A 70-kg 70-kg adult can die from injection of ~100 ng ~ 100 ng of botulinum neurotoxin
Question:
A 70-kg 70-kg adult can die from injection of ~100 ng ~ 100 ng of botulinum neurotoxin or by inhaling -1 ug ~ 1 ug of the toxin. A sensitive assay for the neurotoxin can detect -200pg/mL~200pg/niL in 60 μL60 μL of milk or juice.
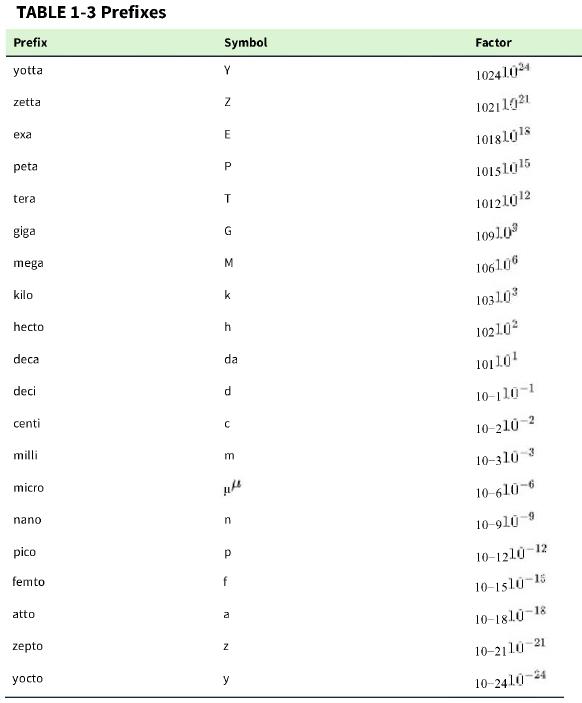
a. How many moles of neurotoxin (molecular mass 150000 g/mol) 150000 g/mol) are in 60 μL60 μL containing 200 pg/mL? 200 pg/mL? Express your answer with a prefix from Table 1-3.
b. How many molecules are in 100ng 100ng of neurotoxin?
Transcribed Image Text:
TABLE 1-3 Prefixes Prefix yotta zetta exa peta tera giga mega kilo hecto deca deci centi milli microf nano pico femto atto zepto yocto Symbol Y N E P T G M k h da d с m n a f a Z y Factor 10241024 10211021 10181018 10151015 10121012 1091.0 1061076 1031.03 10210² 10110¹ 10-110-1 10-210-2 10-310-3 10-610-6 10-910-9 10-1210-12 10-1510-15 10-1810-18 10-2110-21 10-2410-24
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Answer a The amount of neurotoxin in ...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
A chunk of ice of mass ml = 100 g at a temperature tx = 0 C was placed in a calorimeter in which water of mass m1 = 100 g was at a temperature t1. Assuming the heat capacity of the calorimeter to be...
-
How many molecules are present in 4.61 102 mol of O2?
-
How many molecules are present in 2.509 mol of H2S?
-
A study in Batu Pahat General Hospital's on patient account division has compiled data on the age of accounts receivables. The data collected indicates that the age of the accounts follows a normal...
-
In which of the following situations is an information asymmetry likely to cause problems? a. Parents know more than their children about how to write a good college application. b. People who book...
-
Listing 15.17 BallPane.java using a thread to animate bouncing ball movements. Listing 1 import javafx.animation.KeyFrame; 2 import javafx.animation.Timeline; 3 import...
-
Personality traits and job performance. Refer to the Journal of Applied Psychology (January 2011) study of the determinants of task performance, Exercise 12.94 (p. 770). In addition to x1 =...
-
Part A The common stock of Wilson, Inc. is owned by 20 stockholders who live in several states. Wilsons financial statements as of December 31, 1994 were audited by Doe & Co., CPAs, who rendered an...
-
For many of the EMH tests, it is really a test of a joint hypothesis. Discuss what is meant by this concept. What are the joint hypotheses being tested? First test is EMH
-
Troy Engines, Ltd., manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
How many significant figures are there in the following numbers? a. 1.903 01.903 0 b. 0.039 100.039 10 c. 1.40 x104 1.40 x 10 4
-
The chart shows average CO2 CO 2 emissions for fossil fuel vehicles (gasoline, diesel, and hybrid gas-electric), as well as electric vehicles. The abscissa is mg CO2 CO 2 per mile driven per pound...
-
Cost accountants use the concept of equivalent units of production (EUP) to measure actual production for a period in a process costing environment. Write a memo describing what EUP measures and why...
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
A privative clause is which of the following? a. A privative clause is a provision in a statute attempting to prevent the courts from reviewing the decision of an administrative tribunal. b. A...
-
B.) What is the approximate concentration of free Zn 2+ ion at equilibrium when 1.0010 -2 mol zinc nitrate is added to 1.00 L of a solution that is 1.080 M in OH - . For [Zn(OH) 4 ] 2- , K f = 4.610...
-
Limestone consists mainly of the mineral calcite, CaCO3. The carbonate content of 0.5413 g of powdered limestone was measured by suspending the powder in water, adding 10.00 mL of 1.396 M HCl, and...
-
During the 1980s, the average emission of carbon from burning fossil fuels on Earth was 5.4 petagrams (Pg) of carbon per year in the form of CO2.4 (a) How many kg of C were placed in the atmosphere...
-
During the 1980s, the average emission of carbon from burning fossil fuels on Earth was 5.4 petagrams (Pg) of carbon per year in the form of CO2.4 (a) How many kg of C were placed in the atmosphere...
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...

Study smarter with the SolutionInn App


