A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with
Question:
A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with the equiliborium ratio [acrylate]/[acrylic acid] = 0.75. What is the pH of the solution?
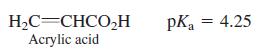
Transcribed Image Text:
H,C=CHCO,H Acrylic acid pKa = 4.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
According to the HendersonHassel...View the full answer

Answered By

Tapas Kumar Mondal
I did my masters of science in chemistry from Indian Institute of Technology Delhi, India in 2014. After that, I have Join to PhD in Indian Association for the Cultivation of Science, Kolkata. During this time I have teach some students and also helped them to solve chemistry numerical.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Acrylic acid (CH2PCHCO2H) is a precursor for many important plastics. (Ka for acrylic acid is 5.6 10-5.)
-
A solution of formic acid (HCOOH) has a pH of 2.53. How many grams of formic acid are there in 100.0 mL of the solution?
-
A 0.10 M solution of a base has pH = 9.28. Find Kb.
-
A rectangular loop of wire 24 cm by 72 cm is bent into an L shape, as shown in FIGURE 23-49. The magnetic field in the vicinity of the loop has a magnitude of 0.035 T and points in a direction...
-
This year, Sam's employer, a privately held company, gave Sam ten shares of stock as a way of saying thank you for all his hard work. The fair market value for the ten shares totals $10,000. Sam paid...
-
Nancy owed Sharon $1,500, but Sharon did not initiate a lawsuit to collect the debt within the time period prescribed by the statute of limitations. Nevertheless, Nancy promises Sharon that she will...
-
What is the difference in accounting for a cash flow hedge and a fair value hedge? LO9
-
What do you think are the sources of sustained superior profitability?
-
! Required information [ The following information applies to the questions displayed below. ] Raner, Harris and Chan is a consulting firm that specializes in information systems for medical and...
-
1. Draw a network diagram that summarizes the systems described above; devices used by administrators, staff, instructors, and students; and the network connections among them. Assume that the...
-
A solution contains 63 different conjugate acid-base pairs. Among them is acrylic acid and acrylate ion, with the equiliborium ratio [acrylate]/[acrylic acid] = 0.75. What is the pH of the solution?
-
Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L. Glycine amide C;H&N20 H,N. FM 74.08 NH,
-
Determine the change in the enthalpy of air, in kJ/kg, as it undergoes a change of state from 100 kPa and 20C to 600 kPa and 300C using the equation of state P(v a) = RT where a = 0.10 m 3 /kg, and...
-
4. (7%) Problem 4: Consider a 570 nm light falling on a single slit of width 1.1 m. Randomized Variables =570 nm w=1.1 um Forbes, David david.forbes@doane.edu @theexpertta.com - tracking id:...
-
(b) The following results are obtained in a double-slit experiment using light from a helium-neon gas laser: Width of 15 fringes = 3.0 cm Separation of slits = 1.5 mm Slit-to-screen distance = 2.5 m...
-
Read the mini-case, Ben and Jerry's Corporate Activism, and answer the following question: What are the pros and cons of Ben and Jerry's political activism when compared to other corporate political...
-
TOPIC : PROBLEMATIZATION - SECOND CURVE THINKING 1. What is second curve thinking?( a More in depth explanation ) 2. What are the implicit assumptions of second curve thinking? ( a More in depth...
-
I think the Power Distance measure in Hofstede's model (Hofstede Insights, n.d.) is particularly interesting.I led divisions in the U.S., New Zealand, and Thailand.Those three countries represented a...
-
Amorphous alloys have been found to have superior corrosion resistance. Corrosion Science (Sept. 1993) reported on the resistivity of an amorphous ironboronsilicon alloy after crystallization. Five...
-
Prairie Outfitters, Inc., a retailer, accepts paymnent through credit cards. During August, credit card sales amounted to $12,000. The processor charges a 3% fee. Assuming that the credit card...
-
A cycloid is the curve described by a point P on the circumference of a circular wheel of radius r rolling along the x axis. The curve is described in parametric form by the equations Use these...
-
Afence around a eld is shaped as shown in Figure P25. It consists of a rectangle of length L and width W and a right triangle that is symmetric about the central horizontal axis of the rectangle....
-
The four-sided gure shown in Figure P26 consists of two triangles having a common side a. The law of cosines for the top triangle states that a 2 = b 2 1 + c 2 1 - 2b 1 c 1 cos A 1 and a similar...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


