A solution of NaOH was standardized by titration of a known quantity of the primary standard, potassium
Question:
A solution of NaOH was standardized by titration of a known quantity of the primary standard, potassium hydrogen phthalate
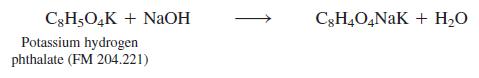
The NaOH was then used to find the concentration of an unknown solution of H2SO4:

(a) Titration of 0.824 g of potassium hydrogen phthalate required 38.314 g of NaOH solution to reach the end point detected by phenolphthalein indicator. Find the concentration of NaOH (mol NaOH/kg solution).
(b) A 10.00-mL aliquot of H2SO4 solution required 57.911 g of NaOH solution to reach the phenolphthalein end point. Find the molarity of H2SO4.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





