a. Use Figure 25-31 to select a tetrahydrofuran/water tetrahydrofuran/water mobile phase strength equivalent to the strength of
Question:
a. Use Figure 25-31 to select a tetrahydrofuran/water tetrahydrofuran/water mobile phase strength equivalent to the strength of 80% 80% methanol.
b. Describe how to prepare 1 liter of this tetrahydrofuran mobile phase.
c. What limitations would be imposed by the use of tetrahydrofuran?
Figure 25-31
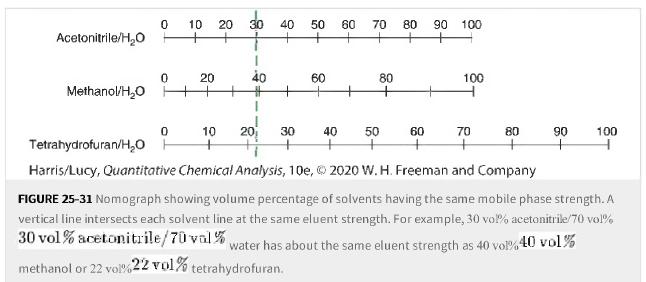
Transcribed Image Text:
Acetonitrile/H₂O 0 10 20 0 Methanol/H₂O + 0 Tetrahydrofuran/H₂O H 20 methanol or 22 vol%4 30 40 40 + # + 1 10 20 30 50 + 60 60 70 80 90 60 80 + + Harris/Lucy, Quantitative Chemical Analysis, 10e, © 2020 W. H. Freeman and Company 80 + + 40 50 100 100 70 90 + 100 FIGURE 25-31 Nomograph showing volume percentage of solvents having the same mobile phase strength. A vertical line intersects each solvent line at the same eluent strength. For example, 30 vol% acetonitrile/70 vol% 30 vol% acetonitrile/70 val% water has about the same eluent strength as 40 vol%40 vol% 22 vol% tetrahydrofuran.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
Answer a To select a tetrahydrofuranwater mobile phase strength equival...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
How to prepare 1 liter of a buffer mixture of pH = 9.5 starting from HCl and 10 mmol of Na2CO3? What will the pH value be after adding to 100 ml of the buffer mixture: a) 5.10-2 mmol of NaOH? b)...
-
This figure shows phase 1 vapor recovery from an underground gasoline storage tank. Before phase 1 vapor recovery was developed, gasoline vapors were vented directly into the air. Now the vapor is...
-
The phase diagram for water is shown in FIGURE 17-37. (a) What is the temperature T1 on the phase diagram? (b) What is the temperature T2 on the phase diagram? (c) What happens to the...
-
Assume you are given the following abbreviated financial statements: On the basis of this information, calculate as many liquidity, activity, leverage, profitability, and common stock measures as you...
-
Use the data in Table 11.6 on page 707. Assume that we wish to fit a simple linear regression model for predicting logarithm of 1980 price from logarithm of 1970 price. a. Find the posterior...
-
List the nine balance-related audit objectives in the verification of the ending balance in inventory and provide one useful audit procedure for each of the objectives.
-
(ii) Is there evidence of two-way interactions between anoxia, root type and genotype? If so, what is the nature of these interactions?
-
On January 2, 2017, TI enters into a contract with Drewry Corp. to build a new piece of equipment. The contract price is $ 3 million, and construction is expected to take 18 months. Drewry is billed...
-
Brief Exercise 7-14 (Part Level Submission) Recent financial statements of General Mills, Inc. report net sales of $12,442,000,000. Accounts receivable are $912,000,000 at the beginning of the year...
-
On January 1, 2012, Port Imports Inc. acquired 90% of the common shares of Spanish Imports Ltd. in exchange for a new issue of its own shares valued at $4,320,000. At that date the shareholders...
-
The figure shows reversed-phase retention data for three compounds. a. Identify whether compounds A, B, and C are weak acids or bases. For each compound, what is the pKap K a and the retention factor...
-
a. UHPLC can provide exquisite resolution when run on long columns at high pressure or rapid separations with reasonable resolution if short columns are run fast. The drug acetaminophen run on a...
-
If the parking attendants can wait on 5 vehicles per minute, the average time T in minutes spent waiting in line and paying the attendant becomes (a) What is a reasonable domain for T? (b) Graph y =...
-
Consider the following C functions and assembly code: int fun4 (int *ap, int *bp) ( int a = *ap; int bbp; return a+b; }) pushl ebp movl esp, ebp int fun5 (int *ap, int *bp) { int bbp; *bp + *ap;...
-
The position of a particle moving along the x-axis is given by x(t) = = 4.2 2.5t m. (Assume t is in seconds.) (a) At what time (in s) does the particle cross the origin? 1.68 S (b) What is the...
-
2. Boxes A and B are being pulled to the right by a rope attached to box B. Box A sits on top of box B, and both boxes accelerate together to the right at a rate of 1.75 m/s. The masses and...
-
You bought a 15-kilogram sack of unshelled peanuts for your restaurant. You weigh the sack three times on a balance, with the following results: Trial Mass (kg) 1 15.02 2 15.49 3 15.91 The results...
-
Two hikers leave the same tent at a campground and go separate ways. One hiker walks 8 miles directly south to Ashville, and the other hiker walks 14 miles directly northwest (i.e., N45W) to...
-
An owl is chasing a squirrel and is using echolocation (the reflection of sound) to aid in the hunt. If the squirrel is 25 m from the owl, how long does it take sound to travel from the owl to the...
-
Suppose the government bond described in problem 1 above is held for five years and then the savings institution acquiring the bond decides to sell it at a price of $940. Can you figure out the...
-
Express the molecular mass ( uncertainty) of C9H9O6N3 with the correct number of significant figures.
-
Express the molecular mass ( uncertainty) of C9H9O6N3 with the correct number of significant figures.
-
Express the molecular mass ( uncertainty) of C9H9O6N3 with the correct number of significant figures.
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


