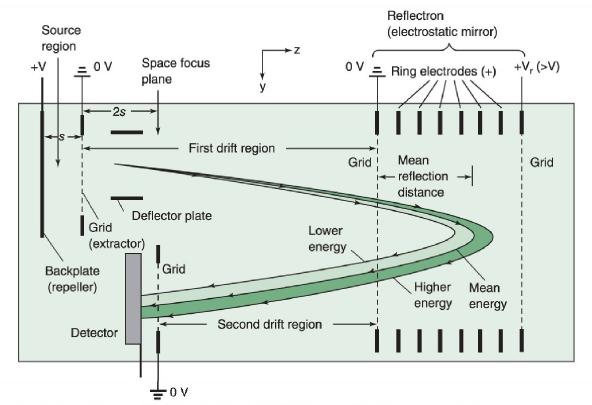
Briefly describe how the time-of-flight mass spectrometer in Figure 22-2 works. What is the benefit of the
Question:
Briefly describe how the time-of-flight mass spectrometer in Figure 22-2 works. What is the benefit of the electrostatic mirror in Figure 22-20?
Figure 22-2

Figure 22-20
Transcribed Image Text:
Grid 1 V₂ = 20 kV to 18 kV Backplate V₂ = 20 kV Sample probe 20 000 V 18 000 V Grid 2 OV Deflector plates Pulsed ultraviolet laser Laser vaporizes samples spot Initial potential Temporary potential after delay time At OVL Backplate Grid 1 20 000 V 18 000 V To high vacuum (10-10 bar) lons with small m/z move faster than ion with large miz -2-m-long field-free drift region Laser pulse Grid 1 potential Af = 0.2-2 μs 10 μs ion Lower ejection grid 1 potential Microchannel plate ion detector -200 V Grid OV Restore grid 1 potential Grid 2 Time Harris/Lucy, Quantitative Chemical Analysis, 10e, © 2020 W. H. Freeman and Company FIGURE 22-2 Schematic diagram of time-of-flight mass spectrometer with matrix-assisted laser desorption/ionization (MALDI) sample introduction and time-delayed ion extraction. -2 kV Gate closed
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The timeofflight TOF mass spectrometer in Figure 222 works by first ionizing the sample to be analyz...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
Briefly describe how the data are used to create profiles of users.
-
Briefly describe how the percentiles are calculated for a data set.
-
Briefly describe how the Bretton Woods system operated.
-
Generate a matrix of random integer temperatures in Fahrenheit from 70 to 100 for 10 weeks (rows) and 7 days per week (columns). The result should look something like this. Assume the first column is...
-
Suppose that both the least-squares line and the least squares parabola were fitted to the same set of points. Explain why the sum of the squares of the deviations of the points from the parabola...
-
Figure Q93 shows a series of modified fluorescent proteins that emit light in a range of colors. How do you suppose the exact same chromophore can fluoresce at so many different wavelengths? Figure...
-
(e) Obtain estimates of the variance components for the same model terms and for the residual term, from the anova. Confirm your answers from the mixed modelling results.
-
Summer Tyme, Inc., is considering a new three-year expansion project that requires an initial fixed asset investment of $3.9 million. The fixed asset will be depreciated straight-line to zero over...
-
ABC Company has a debt ratio of 45%. Suppose the intangible assets of the company represents 10% of total assets, the debt to tangible net worth ratio for the company is:
-
Melodic Musical Sales, Inc. is located at 5500 Fourth Avenue, City, ST 98765. The corporation uses the calendar year and accrual basis for both book and tax purposes. It is engaged in the sale of...
-
The absorbance of a 2.31 x 10 -5 M 2.31x10 -5 M solution of a compound is 0.822 0.822 at a wavelength of 266 nm 266 nm in a 1.000-cm 1.000-cm cell. a. Calculate the molar absorptivity at 266 nm.266...
-
A 100-mL 100-mL sample of hard water containing magnesium and calcium was titrated as illustrated in Figure 18-10. It required 14.59 mL 14.59 mL of 10.83 mM 10.83 mM ethylenediaminetetraacetic acid...
-
Is it fair to utilize the least expensive capital resources, no matter where they come from? Why?
-
Encouraging you to sit back and watch a full hour of one of your favorite shows on prime-time television. However, instead of getting up during the commercial break or fast forwarding through the...
-
A family member has been recently diagnosed with a heart condition that requires replacing a heart valve. She points out that if she goes to India, the surgery cost is about 60% cheaper on average...
-
Based on the case of Bowers Machine Parts. Critically analyze why people were not doing their best and critically explain why hiring a consultant might solve the issue. Justify your answer by using...
-
Identify a few strategies for sustainability effectiveness. Should sustainability be a corporation's top priority? Why or why not? What are the challenges associated with implementing sustainable...
-
Answer the following questions for the topic you want to write about. Type your answers in a separate Word document. What is the issue or debatable idea you might write about? What is debatable about...
-
When a police car is at rest, its siren has a frequency of 750 Hz. You are a thief, and just after finishing a nights work, you hear a siren with a frequency of 700 Hz. Your fellow thief tells you to...
-
Hardin Services Co. experienced the following events in 2016: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
(a) Following the example of ammonia in Section 7-5, write the equilibria and charge and mass balances needed to find the composition of 0.01 M sodium acetate, which you should abbreviate as Na+A-....
-
Calculate the ionic strength of (a) 0.0087 M KOH and (b) 0.0002 M La(IO3)3 (assuming complete dissociation at this low concentration and no hydrolysis reaction to make LaOH2+).
-
Calculate the ionic strength of (a) 0.0087 M KOH and (b) 0.0002 M La(IO3)3 (assuming complete dissociation at this low concentration and no hydrolysis reaction to make LaOH2+).
-
There is a credit rating agency for businesses that gives out various amounts of information based on the subscription level. This company is called a. Business Credit Scoring b. Fair Issue c. Dun...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...

Study smarter with the SolutionInn App


