Question: Calculate the activity coefficient of Al 3+ when = 0.083 M by linear interpolation in Table 7-1. Table 7-1 Ion size Ionic strength (p.
Calculate the activity coefficient of Al3+ when µ = 0.083 M by linear interpolation in Table 7-1.
Table 7-1
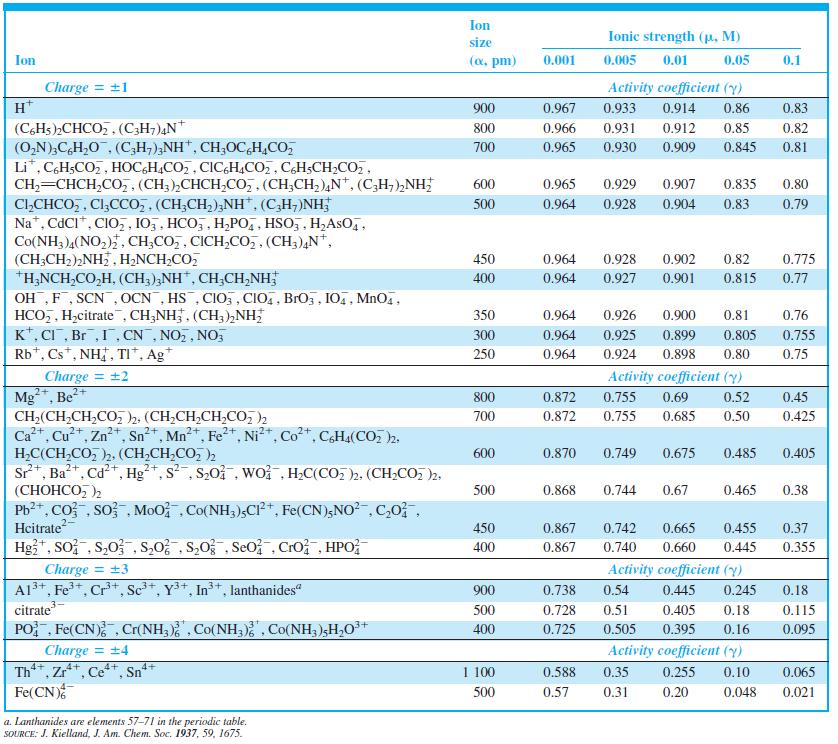
Ion size Ionic strength (p. M) Ion (a, pm) 0.001 0.005 0.01 0.05 0.1 Charge = +1 H* Activity coefficient (y) 900 0.967 0.933 0.914 0.86 0.83 (CHs)2CHCO,,(C;H;),N* (O,N),C,H,0, (C,H;),NH", CH;OC,H,CO, Li*, C,H;CO,, HOCGH,CO,, CIC,H,CO,, CH;CH;CO,, CH,=CHCH,CO,, (CH;),CHCH,Co,, (CH;CH,),N*, (C,H,),NH; CI,CHCO,, Cl;CCo, , (CH;CH,),NH", (C,H;)NH, Na*, CdCI*, CIO,, 105, HCO,, H,PO,, HSO,, H,AsO,, Co(NH3 )4(NO,), CH;CO,, CICH,CO,, (CH3),N*, (CH;CH2),NH, H2NCH,CO, *H,NCH,CO,H, (CH;);NH, CH,CH,NH; OH,F, SCN, OCN, HS , CIO, , CIO,, BrOz, IO,, MnO4, HCO,, H,citrate , CH;NH, (CH3),NH; K*, CI, Br,1, CN , NO,, NO, Rb*, Cs*, NH, TI*, Ag* 800 0.966 0.931 0.912 0.85 0.82 700 0.965 0.930 0.909 0.845 0.81 600 0.965 0.929 0.907 0.835 0.80 500 0.964 0.928 0.904 0.83 0.79 450 0.964 0.928 0.902 0.82 0.775 400 0.964 0.927 0.901 0.815 0.77 350 0.964 0.926 0.900 0.81 0.76 300 0.964 0.925 0.899 0.805 0.755 250 0.964 0.924 0.898 0.80 0.75 Charge = 2 Mg**, Be?+ CH2(CH;CH,CO 2. (CH;CH,CH,CO, )2 Ca+. Cu*, Zn*, Sn*, Mn*, Fe2+, Ni*, Co*, CHa(CO, )2. Activity coefficient (y) 800 0.872 0.755 0.69 0.52 0.45 700 0.872 0.755 0.685 0.50 0.425 0.870 H,C(CH,CO, 2. (CH,CH,CO, ), Sr*, Ba*, Cd+, Hg*, s, S20. wo , H,C(CO, )2, (CH2CO, )2, (, ) Pb2*, Co , so Moo , Co(NH,),CP*, Fe(CN);NO?, C,0, Hcitrate Hg*, So; , S,0 , S,0; , S,0 , Seo Cro, HPO 600 0.749 0.675 0.485 0.405 500 0.868 0.744 0.67 0.465 0.38 450 0.867 0.742 0.665 0.455 0.37 +1 400 0.867 0.740 0.660 0.445 0.355 Charge = 3 A13+, Fe+, Cr+, Sc*, Y3+, In+, lanthanides" citrate PO Fe(CN) , Cr(NH, Co(NH,) , Co(NH, )H,O** Charge = 4 Th*, Zr**, Ce**, Sn* Fe(CN) Activity coefficient (y) 900 0.738 0.54 0.445 0.245 0.18 500 0.728 0.51 0.405 0.18 0.115 400 0.725 0.505 0.395 0.16 0.095 Activity coefficient (y) 4+ 4+ 4+ 4+ 1 100 0.588 0.35 0.255 0.10 0.065 500 0.57 0.31 0.20 0.048 0.021 a. Lanthanides are elements 57-71 in the periodic table. SOURCE: J. Kielland, J. Am. Chem. Soc. 1937, 59, 1675.
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
Hence Value of Activity Coefficient When is 0083 M is equal to 0 20 21 Here ... View full answer

Get step-by-step solutions from verified subject matter experts


