Consider the following equilibria in aqueous solution: (a) Calculate the numerical value of the equilibrium constant for
Question:
Consider the following equilibria in aqueous solution:
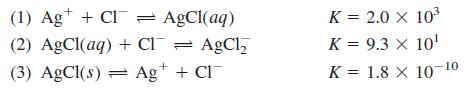
(a) Calculate the numerical value of the equilibrium constant for the reaction
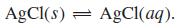
(b) Calculate the concentration of AgCl(aq) in equilibrium with excess undissolved solid AgCl.
c) Find the numerical value of K for the reaction
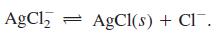
Transcribed Image Text:
(1) Ag* + Cl = AgCl(aq) (2) AgCl(aq) + Cl¯ = AgCl, K = 2.0 X 103 %3D K = 9.3 x 10' (3) AgCl(s) = Ag+ + Cl K = 1.8 X 10-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
a The overall reaction is Ag Cl Ag Cl We can write the equilibrium expression for this reaction as K ...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider the equilibrium Calculate the equilibrium constant Kp for this reaction, given the following information (at 298 K):
-
Consider the system A(g) B(g) at 25oC. a. Assuming that GoA = 8996 J/mol and GoB = 11,718 J/ mol, calculate the value of the equilibrium constant for this reaction. b. Calculate the equilibrium...
-
Consider the following reaction run at standard conditions: Al(s) + Fe2+(aq) Fe(s) +Al3+(aq) a. Calculate the standard cell potential for this cell from standard free energies of formation (see...
-
Assume that you are purchasing shares in a company in the variety store and gas bar supply business. Suppose you have narrowed the choice to BFI Trading Ltd. and Lin Corp. and have assembled the...
-
Sue is 30 years old and is president and a 51% shareholder of C Corporation. She informs you that C Corporation has 10 shareholders, all unrelated. Other than herself, no shareholder owns more than...
-
(Module 2 only) Mini Review: Consolidation (no worksheet): Equity Method The following six accounts appear on the separate company financial statements (as opposed to a trial balance) of a parent and...
-
A firms stock earns $2 per share, and the firm distributes 40 percent of its earnings as cash dividends. Its dividends grow annually at 4 percent. a) What is the stocks price if the required return...
-
At the various activity levels shown, Yates Company incurred the following costs: Required Identify each of these costs as fixed, variable, ormixed. Units Sold 20 40 60 80 100 a. Total cost of...
-
Pharoah Company sells one product. Presented below is information for January for Pharoah Company. Nov. 1 Inventory 270 units at $ 10 each 5 Purchase 170 units at $ 11 each 10 Sale 370 units at $ 20...
-
1. How has a commitment to corporate values contributed to Whole Foodss success? 2. Describe how Whole Foodss adoption of a stakeholder orientation has influenced the way it operates. 3. What are...
-
The U.S. Department of Agriculture provided homogenized baby food samples to three labs for analysis.3 Results agreed well for protein, fat, zinc, riboflavin, and palmitic acid. Results for iron were...
-
Reaction 6-7 is allowed to come to equilibrium in a solution initially containing 0.0100 M BrO - 3 , 0.0100 M Cr 3+ , and 1.00 M H + . To find the concentrations at equilibrium, we can construct a...
-
Compute (a) The characteristic polynomial of A, (b) The eigenvalues of A, (c) A basis for each eigenspace of A, (d) The algebraic and geometric multiplicity of each eigenvalue. 3 1 0 0 A = 0 0 1 1
-
inverse function of f ( x ) = 9 - 8 e ^ x
-
Let = <3,2,-1) = < 1,3 -> W=
-
1. This is a group assignment, and the lecturer will create and finalize assignment groups in week 3/4. (4-5 members in each group). 2. Identify a problem (only one problem relating to OB) in an...
-
Fromthefollowinginformation, preparejournalentriestodistributetransportationexpenses(ontheaverage rate permilepermonthmethod)andstoresexpenses. Truckmileageduringthemonth:...
-
2 Staffing at the Optimal Utilization A large theme park is attempting to staff its check-in desks. Currently, the arrival rate is A = 364.5 customers per hour, and each server can check-in p=81...
-
Refer to the Human Factors (Dec. 1988) study of color brightness as a body orientation clue, Exercise 7.65. Ninety college students, reclining on their backs in the dark, were disoriented when...
-
on 8 For the following set of lengths 130, 170, 160, 160, 150, 190 Third quartile is: et red d out of Select one: O a. 160 a question O b. 145 O c. 175 O d. 180
-
Fractional composition in a tetraprotic system. Prepare a fractional composition diagram analogous to Figure 9-4 for the tetraprotic system derived from hydrolysis of Cr3+: Cr3+ + (H2O) Cr(OH)2+ +...
-
What is the difference between the isoelectric pH and the isoionic pH of a protein with many different acidic and basic substituents?
-
Consider the diprotic acid H2A with K1 = 1.00 10-4 and K2 = 1.00 10-8. Find the pH and concentrations of H2A, HA-, and A2- in (a) 0.100 M H2A; (b) 0.100 M NaHA; (c) 0.100 M Na2A.
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


