Histidine is a triprotic amino acid: What is the value of the equilibrium constant for the reaction
Question:
Histidine is a triprotic amino acid:
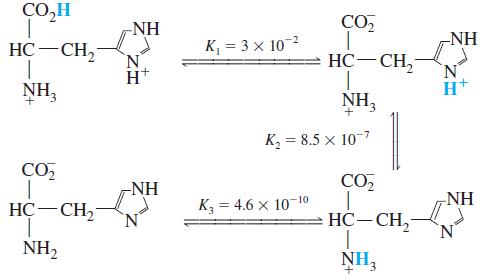
What is the value of the equilibrium constant for the reaction
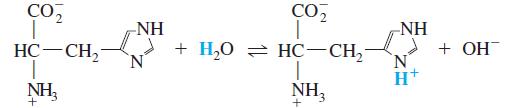
Transcribed Image Text:
CO,H -NH CO, -NH HC-CH, K, = 3 x 102 HC-CH, NH, NH, к, 3 8.5 х 10-7 CO, -NH CO NH HC-CH2 К, 3 4.6 х 10 10 HC-CH, N. NH2 NH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
Using definition of equilibrium constant Loo...View the full answer

Answered By

Amit Kulhria
I have been working as Chemistry professor while training students for JEE Main and Advanced for about 7 years. Besides I have taught general science to Civil services aspirants.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Write the stepwise acid-base reactions for the following ions in water. Write the correct symbol (for example, Kb1) for the equilibrium constant for each reaction. a. b. H3NCH2CH2NH...
-
The amino acid glycine (H2N-CH2-COOH) can participate in the following equilibria in water: (a) Use the values of Ka and Kb to estimate the equilibrium constant for the intramolecular proton transfer...
-
What average yield per amino acid would be required to synthesize a protein containing 100 amino acids in 507% overall yield?
-
Explain the concept of recursion in programming and provide an example of a recursive function.
-
How are Treasury regulations and revenue rulings different?
-
On August 10, 2016, Theta Electronic Laboratories, Inc., executed a promissory note to George and Marguerite Thomson. Six other individuals, Gerald Exten, Emil ONeil, James Hane, and their wives also...
-
Based on the information that you derived, would you sell any of the ten stocks that you selected?
-
Contrast the strengths of bottom-up and top-down approaches to project budgeting.
-
On January 1, 2020, QuickPort Company acquired 90 percent of the outstanding voting stock of NetSpeed, Inc., for $909,000 in cash and stock options. At the acquisition date, NetSpeed had common stock...
-
a. A notch filter is required to be designed, that produces a null at 5 kHz and unity gain at dc. The filter operates at a sampling frequency of 20 kHz. b. A continuous time signal is given to the...
-
Succinic acid dissociates in two steps: K1 H,H,C ,, + H* %3| || OCCH,CH,CO + H* , 3 2.3 10-6 HOCCH,CH,CO Calculate Kp1 and Kp2 for the following reactions: || OCCH,CH,CO + H,0 = HOCCH,CH,CO + OH ...
-
(a) From Kw in Table 6-1, calculate the pH of pure water at 0, 20, and 40C. (b) For the reaction D 2 O D + + OD - , K = [D + ][OD - ] =1.35 10 -15 at 25C. In this equation, D stands for deuterium,...
-
Why should a mission statement be relatively short? Appendix
-
Consider the following C functions and assembly code: int fun4 (int *ap, int *bp) ( int a = *ap; int bbp; return a+b; }) pushl ebp movl esp, ebp int fun5 (int *ap, int *bp) { int bbp; *bp + *ap;...
-
The position of a particle moving along the x-axis is given by x(t) = = 4.2 2.5t m. (Assume t is in seconds.) (a) At what time (in s) does the particle cross the origin? 1.68 S (b) What is the...
-
2. Boxes A and B are being pulled to the right by a rope attached to box B. Box A sits on top of box B, and both boxes accelerate together to the right at a rate of 1.75 m/s. The masses and...
-
You bought a 15-kilogram sack of unshelled peanuts for your restaurant. You weigh the sack three times on a balance, with the following results: Trial Mass (kg) 1 15.02 2 15.49 3 15.91 The results...
-
Two hikers leave the same tent at a campground and go separate ways. One hiker walks 8 miles directly south to Ashville, and the other hiker walks 14 miles directly northwest (i.e., N45W) to...
-
Show that Note that since (x i - x) = 0. T(x - I) Bo = y - Bx = 2
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
Include activity coefficients from the Davies equation to find the pH and concentrations of species in the mixture of sodium tartrate, pyridinium chloride, and KOH in Section 12-1. Consider only...
-
(a) Using the ion-pair equilibrium constant from Appendix J, with activity coefficients = 1, find the concentrations of species in 0.025 M MgSO 4 . Hydrolysis of the cation and anion near neutral pH...
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...
-
Determine the simple interest earned on $10,000 after 10 years if the APR is 15%
-
give me an example of 10 transactions from daily routine that we buy and put for me Liabilities + Owners' Equity + Revenues - Expenses

Study smarter with the SolutionInn App


