Some bath salts are synthetic cathinones designed to circumvent laws, but have unknown properties and side effects.
Question:
Some “bath salts” are synthetic cathinones designed to circumvent laws, but have unknown properties and side effects. The acid dissociation constant affects the toxicology of the derivatives. Electrophoretic mobility can be used to determine the pKapKa of a compound.
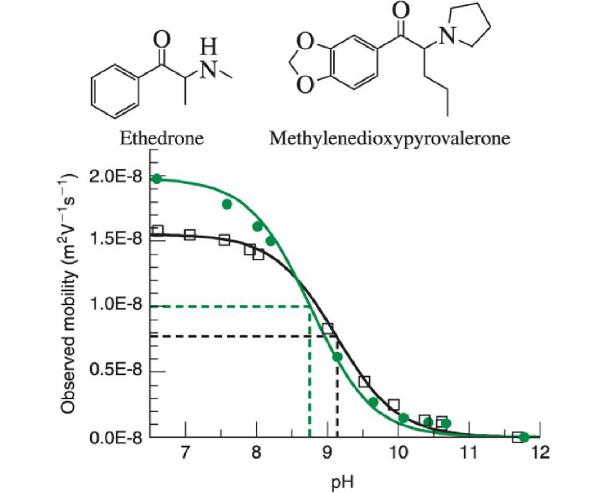
a. For weak bases like ethedrone and methylenedioxypyrovalerone,
Observed electrophoretic mobility:![]() Use this expression to explain the shape of the plot of observed electrophoretic mobility versus pH.pH.
Use this expression to explain the shape of the plot of observed electrophoretic mobility versus pH.pH.
b. Electrophoretic mobility is proportional to the charge of the ion and inversely proportional to the size of the ion.
Which fully protonated cathinone would be expected to have a greater electrophoretic mobility?
c. Based on the electrophoretic mobility of the fully protonated base (μepBH+),(μepBH+), which curve is for ethedrone and which is for methylenedioxypyrovalerone?
d. The pKapKa values for the two cathinones are 8.778.77 and 9.109.10 on the graph. Which acid dissociation constant is for ethedrone?
e. What pHpH would provide greatest resolution between the two cathinones?
Step by Step Answer:

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy





