Using activities, find the concentrations of the major species in 0.10 M NaClO 4 saturated with Mn(OH)
Question:
Using activities, find the concentrations of the major species in 0.10 M NaClO4 saturated with Mn(OH)2. Take the ionic strength to be 0.10 M and suppose that the ion size of MnOH+ is the same as Mn2+. Consider just the following chemistry:
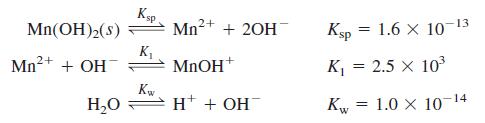
Transcribed Image Text:
Ksp Mn2+ 13 Mn(OH)2(s) + 20H Ksp = 1.6 X 10 Mn2+ + OH K, MNOH* K = 2.5 x 103 К H* + OH 1.0 x 10- Kw -14 H,0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
Or H Mn OHS Ksp Mn 2OH Or H Ksp 161013 Mn OHK Mn ...View the full answer

Answered By

Saurav Solanki
I love physics . After i did btech(grad) in electronics , started teaching physics as a home tutor , later in 2017 i satrted tutoring online. I have taught in Chegg , 24houranswers.com . Learning physics concept is easy when taught with practical examples . With my 3 yrs of experience , i want you to give me chance to teach you in a better way.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use the systematic treatment of equilibrium to find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions: LiF(s) = Li* +F Ksp = [Li*]YLi+[F]YF Kjon...
-
Find the concentrations of Ag+(aq), NH3(aq), and [Ag(NH3)2]+(aq) at equilibrium when 0.10 mol Ag+(aq) and 0.10 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for the...
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
At its headquarters in Ventura, California, Patagonia's office space feels more like a national park lodge than the main office of a $400 million retailer. It has a Douglas fir staircase and a...
-
A developer acquired a parcel of unimproved real property that she would like to develop. Although the land is currently zoned for commercial use, the developer would prefer not to begin development...
-
Define each of these terms, and indicate how each is determined. a. Expected activity time. b. Variance of an activity time. c. Standard deviation of a paths time. LO.1
-
Comparing Housing Alternatives. Interview several people about the factors that influenced the selection of their current residence. Also ask about features that might be available in homes in the...
-
Life Is Good Financial Services has provided Good Things to Eat Groceries a proposal for in-store branches in two of its four Davidson locations. Good Things to Eat counter proposed opening one...
-
Determine the amount of dividends paid each year to each of the two classes of stockholders assuming that the preferred stock is cumulative. Also determine the total dividends paid to each class for...
-
1. Using the spreadsheet model from Case 2.1 as a starting point, use Solver to find the optimal set of projects to approve. The solution should maximize the total NPV from the approved projects, and...
-
Write charge and mass balances for aqueous Ca 3 (PO 4 ) 2 if the species are Ca 2+ , CaOH + , CaPO - 4 , PO 3- 4 , HPO 2 4 - , H 2 PO - 4 , and H 3 PO 4 .
-
Explain why the solubility of an ionic compound increases as the ionic strength of the solution increases (at least up to ~ 0.5 M).
-
A cross-channel shopper is an online consumer who researches products online and then purchases them at a retail store. These shoppers are reached through multichannel marketing. Websites play a...
-
At the Business Level there are a couple main strategies that companies use- Cost Leadership and Differentiation. What is the difference between them? Share some examples of companies or specific...
-
https://youtu.be/c_Eutci7ack After watching the video, what are your thoughts on Power? Would you want to have this Power ? Why would you not want this Power? If you are a manager or want to be a...
-
Find anti derivative of ( 2 t - 4 + 3 ^ ( 1 / 2 ) ) / t ^ ( 1 / 2 )
-
How Adidas is using creative narratives to build brand equity Adidas' outdoor division is drawing on the expertise of its wider athletic business while at the same time flexing its creative muscle to...
-
May I have a word" Alysha Stark popped her head in at the corner office of the Managing Director Mike O' Connor. It's early on a Monday morning. When Alysha, his star Director, starts something this...
-
Ecologists classify the cause of forest fragmentation as either anthropogenic (i.e., due to human development activities such as road construction or logging) or natural in origin (e.g., due to...
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
Calculate pCo2+ at each of the following points in the titration of 25.00 mL of 0.020 26 M Co2+ by 0.03855 M EDTA at pH 6.00: (a) 12.00 mL; (b) Ve; (c) 14.00 mL.
-
Consider the titration of 25.0 mL of 0.0200 M MnSO4 with 0.010 0 M EDTA in a solution buffered to pH 8.00. Calculate pMn2+ at the following volumes of added EDTA and sketch the titration curve: (a) 0...
-
For the same volumes used in Problem 11-8, calculate pCa2+ for the titration of 25.00 mL of 0.02000 M EDTA with 0.010 00 M CaSO4 at pH 10.00.
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


