Question: Using the linear calibration curve in Figure 4-13, find the quantity of unknown protein that gives a measured absorbance of 0.264 when a blank has
Using the linear calibration curve in Figure 4-13, find the quantity of unknown protein that gives a measured absorbance of 0.264 when a blank has an absorbance of 0.095.
Figure 4-13
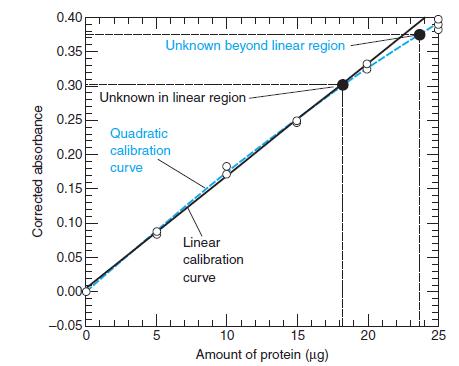
0.40 Unknown beyond linear region 0.35 0.30 Unknown in linear region 0.25 Quadratic 0.20 calibration curve 0.15 0.10 Linear 0.05 calibration curve 0.000 -0.05 0. 10 15 20 25 Amount of protein (ug) Corrected absorbance
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Assuming that the linear calibration curve in Figure 413 is valid for the range of protein concentra... View full answer

Get step-by-step solutions from verified subject matter experts


