What is the purpose of the WO3 WO 3 and Cu Cu in Figure 27-8? Figure 27-8
Question:
What is the purpose of the WO3WO3 and CuCu in Figure 27-8?
Figure 27-8
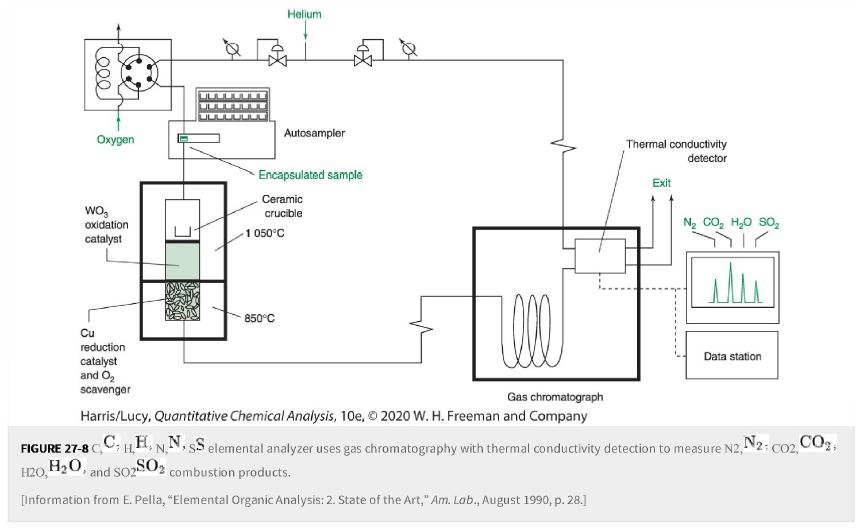
Transcribed Image Text:
Leee Oxygen WO oxidation catalyst Cu reduction catalyst and O₂ scavenger 7. Encapsulated sample Ceramic crucible Helium Autosampler 1 050°C 850°C Well Gas chromatograph Harris/Lucy, Quantitative Chemical Analysis, 10e, © 2020 W. H. Freeman and Company Thermal conductivity detector FIGURE 27-8 C H20, H₂O, and S02 SO2 combustion products. [Information from E. Pella, "Elemental Organic Analysis: 2. State of the Art," Am. Lab., August 1990, p. 28.] Exit Ng CO, HO SO, Sair Data station CHEN. HH, NN, SS elemental analyzer uses gas chromatography with thermal conductivity detection to measure N2, N2: CO2, CO₂,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
The WO3WO3 and CuCu combination is placed in a single process in ord...View the full answer

Answered By

Tamondong Riza
Professionally, I am a teacher with years of experience tutoring math and science, as well as teaching in both public schools and independent schools. I feel that education should be an enlightening experience for all children, and I'm committed to helping my students learn new skills and make progress in their subjects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
What is the purpose of IFRS 8 on Operating Segments?
-
What is the purpose of application controls? How does it differ from the purpose of general controls?
-
What are transportation user charges? What is the purpose of such charges?
-
Elmira, Inc had $20,000,000 of bonds outstanding on Dec 31, 2018. The ten year bonds were issued on Jan 1, 2012 for $21,000,000. Elmira can call the bonds at 102% any time after Jan 1, 2018. At the...
-
Consider a multiple linear regression problem with design matrix Z and observations Y. Let Z1 be the matrix remaining when at least one column is removed from Z. Then Z1 is the design matrix for a...
-
Arrange the following substances in order of increasing solubility in hexane, C 6 H 14 : CH 2 OHCH 2 OH, C 10 H 22 , H 2 O.
-
2. Compute all first-order partial derivatives of each of the following functions and find where they are continuous. (a) f(x,y) = x2 +sin(xy). (b) f(x,y,z) =~. (c) f(x,y) = Jx2 +y2. l+z
-
Larry has severe vision problems and, in the past, he has claimed the additional standard deduction available to blind taxpayers. This year Larrys doctor prescribed a new type of contact lens that...
-
Because of increasing global demand for its fiber optics products, Hanover Inc. is currently facing a decision on how to increase its production capacity. Hanover has three fiber optic products...
-
The company Smart Inc. is a company that produces Dog Shampoo in Toronto area. The results of the company, which has been mediocre for the past couple of years, have been presented in the annual...
-
Write a balanced equation for combustion of C 8 H 7 NO 2 SBrCl C 8 H 7 NO 2 SBrCl in a C,C,H,H,N,N,SS elemental analyzer.
-
Corrected peak area. A micellar electrokinetic chromatography method for phthalazine and its metabolite 1-1- phthalazinone yielded the following repeatability for six replicate injections of a 50 M...
-
Complete and balance the equations below, and classify them as precipitation, acidbase, gasforming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) NiCO3 +...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
Identifying Binomial Distributions. Determine whether the given procedure results in a binomial distribution or a distribution that can be treated as binomial (by applying the 5% guideline for...
-
Case 6: TOMS Shoes in 2016: An Ongoing Dedication to Social Responsibility, by Margaret A. Peteraf, Sean Zhand, and Meghan L. Cooney (page C-57) Read the case and then respond to the case questions...
-
Quatro Co. issues bonds dated January 1, 2019, with a par value of $740,000. The bonds' annual contract rate is 13%, and interest is paid semiannually on June 30 and December 31. The bonds mature in...
-
Wildcat Mining wants to know the appropriate discount rate to use in their capital budgeting decision making process. Based on the following data, what is the weighted average cost of capital the CFO...
-
Two adjacent notes in the musical scale used in Western music have fundamental frequencies whose ratio is approximately 1.059. For example, the fundamental frequencies of the notes C-sharp and C have...
-
(a) Bright Sdn Bhd (BSB) is a tax resident manufacturing company in Johor, which involves in ceramic tiles. Currently, BSBs annual sales turnover has been forecasted to be around RM 300,000 for the...
-
(a) Use the ideal gas law (Problem 1-16) to calculate the density (g/mL) of helium at 20C and 1.00 bar. (b) Find the true mass of Na (density = 0.97 g/mL) weighed in a glove box with a He atmosphere,...
-
(a) The equilibrium vapor pressure of water at 20C is 2330 Pa. What is the vapor pressure of water in the air at 20C if the relative humidity is 42%? (Relative humidity is the percentage of the...
-
Effect of altitude on electronic balance. If an object weighs ma grams at distance ra from the center of the Earth, it will weigh mb = ma(r2a/r2b) when raised to rb. An object weighs 100.0000 g on...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...
-
5. The current spot exchange rate is 0.95/$ and the three-month forward rate is 0.91/$. Based on your analysis of the exchange rate, you are pretty confident that the spot exchange rate will be...

Study smarter with the SolutionInn App


