Complete and balance the equations below, and classify them as precipitation, acidbase, gasforming, or oxidationreduction reactions. Show
Question:
Complete and balance the equations below, and classify them as precipitation, acid–base, gasforming, or oxidation–reduction reactions. Show states for reactants and products (s, ℓ, g, aq).
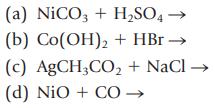
Transcribed Image Text:
(a) NiCO3 + H₂SO4 - (b) Co(OH)2 + HBr → (c) AgCH3CO₂ + NaCl → (d) NIO + CO →
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a NiCO3 s H2SO4 aq NiSO4 aq H2CO3 aq This is an acidbase reaction Carb...View the full answer

Answered By

Lalit Kumar
I love to teach the students.It's just not a profession for me, it is my passion. I had taught many students at the best of my level. I'll give one hundred percent to your problems. Let experience my tutoring skill together.LETS GO.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Complete and balance the equations for the following reactions. a. Li(s) + N2(g) b. Rb(s) + S(s) c. Cs(s) + H2O(l) d. Na(s) + Cl2(g)
-
The following reactions occur in aqueous solution. Complete and balance the molecular equations using phase labels. Then write the net ionic equations. a. CaS + HBr b. MgCO3 + HNO3 c. K2SO3 + H2SO4
-
The following reactions occur in aqueous solution. Complete and balance the molecular equations using phase labels. Then write the net ionic equations. a. BaCO3 + HNO3 b. K2S + HCl c. CaSO3(s) + HI
-
Consider the following actual FY2019 data and a forecast of FY2020 selected balance sheet and income statement numbers. $ millions FY2019 Actual FY2020 Est. $29,009 $32,102 14,592 16,051 8,755 9,923...
-
Figure shows that Japans short-term interest rates have had periods during which they are near or equal to zero. Is the fact that the yen interest rates shown never drop below zero a coincidence, or...
-
The spring is unstretched when = 0. Determine the angle for equilibrium. Due to the roller guide, the spring always remains vertical. Neglect the weight of the links. Given: P = 8 lb K= 50lb/ft a =...
-
Provide an example of a good externality, that is, one that increases a projects true NPV. AppendixLO1
-
Q1. Review the following accounts, subtotals, and totals; (1) describe your observations; and then (2) identify what your observations indicate. A response is given for Cash and Short-term...
-
Data related to the inventories of Costco Medical Supply are presented below: Surgical Equipment Surgical Supplies Rehab Equipment Rehab Supplies Selling price $ 276 $ 131 $ 359 $ 156 Cost 151 100...
-
The products formed in several reactions are given below. Identify the reactants (labeled x and y) and write the complete balanced equation for each reaction. (a) x + y HO(l) + CaBr(aq) (b) x + y ...
-
Balance each of the following equations, and classify them as precipitation, acidbase, gas-forming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) CuCl + HS...
-
If two events are mutually exclusive, then a. their probabilities can be added. b. they may also be collectively exhaustive. c. the joint probability is equal to 0. d. if one occurs, the other cannot...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
(25 Points) University Painting is considering investing in a new paint sprayer to allow them to paint more classrooms in less time. The sprayer would have the following cash flow and cost of capital...
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
The T-accounts below summarize the ledger of Salvador's Gardening Company, Inc. at the end of the first month of operations. Instructions (a) Prepare the journal entries (including explanations) that...
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
A sample of Na2SO4(s) is dissolved in 225 g of water at 298 K such that the solution is 0.325 molar in Na 2 SO 4 . A temperature rise of 0.146C is observed. The calorimeter constant is 330. J K 1 ....
-
Assign a name for each of the following compounds. a. b. c.
-
a. Using the relationships derived in Example Problem 7.1 and the values of the critical constants for water from Table 7.2, calculate values for the van der Waals parameters a, b, and R from z c , T...
-
Need help with every part!!!! Bill Smith is reviewing the cash accounting for Postalman, Inc.a local mailing service. Smith's review will focus on the petty cash account and the bank reconciliation...
-
Janco Industries has a relevant range extending to 30,000 units each month. The following performance report provides information about Janco's budget and actual performance for November. (Click the...
-
On September 18, 2019, Rose Company purchased 11,800 shares (14%) of Wozniak, Inc. stock for $42 per share. The market value of the Wozniak stock at December 31, 2019 was $29 per share. Rose Company...

Study smarter with the SolutionInn App


