The products formed in several reactions are given below. Identify the reactants (labeled x and y) and
Question:
The products formed in several reactions are given below. Identify the reactants (labeled x and y) and write the complete balanced equation for each reaction. 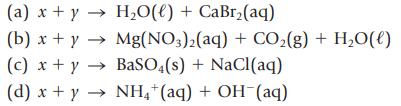
Transcribed Image Text:
(a) x + y → H₂O(l) + CaBr₂(aq) (b) x + y → Mg(NO3)2(aq) + CO₂(g) + H₂O(l) (c) x + y BaSO4(s) + NaCl(aq) → (d) x + y → NH4+ (aq) + OH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a x y H2Ol CaBr2aq Reactants 2 HClaq CaCO3s Balanced Equation 2 HClaq CaCO3s H2Ol C...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The products formed in several reactions are given below. Identify the reactants (labeled x and y) and write the complete balanced equation for each reaction. (a) x + y (NH4)2SO4(aq) (b) x + y...
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
Write a balanced equation for each reaction. (a) (b) (c) (d) H SO, heat CH3 CH2CH-CH NaOC(CH3 3 Br Br Nal CHCH CH-CH acetone NaOH, heat CH3 CH CCH3 Br
-
Jeremiah Wedgewood, the CFO, is adamant that the company needs to move ahead with the new division. While Josey also thinks that creating a new division with a new product line is a good idea, she is...
-
How might a zero interest rate complicate the task of monetary policy?
-
A force P is applied to the end of the lever. Determine the horizontal force F on the piston for equilibrium. B
-
In what ways is the setup for finding a projects cash flows similar to the projected income statements for a new single-product firm? In what ways would the two statements be different? AppendixLO1
-
Do you believe that the $785,000 amount at the center of the Overstock-Grant Thornton dispute was material? Defend your answer. What factors other than quantitative considerations should have been...
-
I am having a hard time with the formulas. So far I have computed only the sales. I have tried looking at examples but I'm not getting the same calculations, can someone please assist me with this...
-
Indicate which of the following copper(II) salts are soluble in water and which are insoluble: Cu(NO3)2, CUCO3, Cu3(PO4)2, CuCl.
-
Complete and balance the equations below, and classify them as precipitation, acidbase, gasforming, or oxidationreduction reactions. Show states for reactants and products (s, , g, aq). (a) NiCO3 +...
-
How will current problems and frustrations be addressed?
-
Notation of 0 + Using the same survey described in Exercise 1, the probability of randomly selecting 50 speaking characters from movies and getting 40 females is expressed as 0+. Does 0+ indicate...
-
A simple random sample of 10 pages from a dictionary is obtained. The numbers of words defined on those pages are found, with the results n = 10, x = 66.4 words, s = 16.6 words. Given that this...
-
Question 3 58.5 Average global temperature 1880-2013 58.0 $ 57.5 57.0 56.5 1880 1900 1920 1940 1960 1980 2000 2020 Year The graph above indicates that global temperatures have Ovaried randomly over...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Selected transactions from the journal of Baylee Inc. during its first month of operations are presented here. Instructions (a) Post the transactions to T-accounts. (b) Prepare a trial balance at...
-
we have to compute the letter grades for a course. The data is a collection of student records stored in a file. Each record consists of a name(up to 20 characters), ID (8 characters), the scores of...
-
Assign a name for each of the following compounds. a. b. c.
-
21.05 g of steam at 373 K is added to 415 g of H 2 O(l) at 298 K at a constant pressure of 1 bar. Is the final state of the system steam or liquid water? Calculate S for the process.
-
A van der Waals gas has a value of z = 1.00061 at 410. K and 1 bar and the Boyle temperature of the gas is 195 K. Because the density is low, you can calculate V m from the ideal gas law. Use this...
-
A company recorded 2 days of accrued tries of 1900 for its employees on January 21 on February, it poids employees Sa 000 for these accrued salaries and for other alones earned through February 9....
-
Need correct answers with workings to this question asap! I've given all the information below, and please follow whatever is given, this all the information I have! Question 4 Icy Delight Company,...
-
Adcock Corp. had $500,000 net income in 2020. For all of 2020, there were 200,000 shares of Adcocks common stock outstanding. There were also 30,000 options outstanding all year. Each option allowed...

Study smarter with the SolutionInn App


