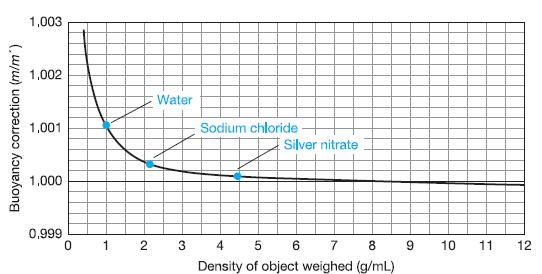
Why is the buoyancy correction equal to 1 in Figure 2-9 when the density of the object
Question:
Why is the buoyancy correction equal to 1 in Figure 2-9 when the density of the object being weighed is 8.0 g/mL?
Figure 2-9
Transcribed Image Text:
1,003 1,002 Water 1,001 Sodium chloride E Silver nitrate 1,000 0,999 1 4 5 6 7 8 10 11 12 Density of object weighed (g/mL) Buoyancy correction (m/m")
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)

Related Book For 

Question Posted:
Related Video
Density helps us predict whether something will float. Density is super important to consider when building things like ships and submarines. The experiment in the video examines the water density by comparing a glass of water containing sugar with a simple glass of water
Students also viewed these Engineering questions
-
Why does buoyancy occur? Under what circumstances does an object float in a fluid?
-
The two strings in FIGURE P17.49 are of equal length and are being driven at equal frequencies. The linear density of the left string is 5.0 g/m. What is the linear density of the right string? www-...
-
The density of quartz mineral was determined by adding a weighed piece to a graduated cylinder containing 51.2 mL water. After the quartz was submerged, the water level was 65.7 mL. The quartz piece...
-
How do the Uniform Trade Secrets Act (UTSA) and the Economic Espionage Act of 1996 differ? Why don't these acts always provide a sufficient remedy for the theft of trade secrets?
-
Early this year, a taxpayer was clearing dry brush from behind his Malibu home in California. He became frustrated with how long it was taking using his clippers. He decided instead to light the...
-
The following represents selected information taken from a companys aging schedule to estimate uncollectible accounts receivable at year end. Instructions (a) Calculate the total estimated bad debts...
-
Which of the following information items with regard to a major customer must be disclosed? a. The identity of the customer. LO3 b. The percentage of total sales derived from the major customer. c....
-
Sue exchanges a sport utility vehicle (adjusted basis of $16,000; fair market value of $19,500) for cash of $2,000 and a pickup truck (fair market value of $17,500). Both vehicles are for business...
-
Cornerstone Exercise 6.6 (Algorithmic) Nonuniform Inputs Apeto Company produces premium chocolate candy bars. Conversion costs are added uniformly. For February, EWIP is 40 percent complete with...
-
1. List in detail the various internal control weaknesses and weaknesses in the monitoring system that existed at the Knottyville Country Club 2. You are hired as an expert CPA to institute effective...
-
After safety features and safety procedures in your laboratory have been explained to you, make a list of them.
-
Pentane (C 5 H 12 ) is a liquid with a density of 0.626 g/mL near 25C. Find the true mass of pentane when the mass in air is 14.82 g. Assume air density = 0.001 2 g/mL.
-
A criminologist studying criminal offenders who have a record of one or more arrests is interested in knowing whether the educational achievement level of the offender influences the frequency of...
-
Country Analysis of Milan: -Discuss the overall cultural, political, legal, economic, and technological infrastructure issues that could potentially impact a product's introduction in Milan. -You...
-
Sarah is a highly introverted employee who works in a fast-paced sales team. Her manager recently noticed that Sarah tends to be reserved during team meetings and rarely volunteers her ideas....
-
Community Disaster Preparedness Review the information found at https://www.ready.gov/community-preparedness-toolkit Look at the steps involved in formulating community response to a disaster....
-
The topic of diversity, tolerance, and inclusion has been widely (and, in many cases, hotly) debated in society over the past several years. In my opinion (and this is up for discussion - hence this...
-
The objective of this coursework is for you to critically engage with the theory and practice of promoting wellbeing or equality, diversity and inclusion (including cultural diversity) for employees...
-
Given a choice between the high-low method, a scattergraph, or regression analysis, which method would you prefer for separating a mixed cost into its fixed and variable components? Why?
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
(a) How many milliliters of 53.4 (0.4) wt% NaOH with a density of 1.52 ( 0.01) g/mL will you need to prepare 2.000 L of 0.169 M NaOH? (b) If the uncertainty in delivering NaOH is [1] 0.01 mL,...
-
We have a 37.0 ( 0.5) wt% HCl solution with a density of 1.18 ( 0.01) g/mL. To deliver 0.050 0 mol of HCl requires 4.18 mL of solution. If the uncertainty that can be tolerated in 0.050 0 mol is 2%,...
-
Compute the molecular mass and its standard uncertainty for NH 3 . What is the percent relative uncertainty in molecular mass?
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


