Question
a. Calculate the energy required to ionize a ground state hydrogen atom. Report your answer in kilo joules. b. What is the longest wavelength of
a. Calculate the energy required to ionize a ground state hydrogen atom. Report your answer in kilo joules.
b. What is the longest wavelength of electromagnetic radiation capable of ionizing a ground state hydrogen atom? Report your answer in nanometers.
c. Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. Report your answer in kilo joules.
d. What is the longest wavelength of electromagnetic radiation capable of ionizing this hydrogen atom in an excited state? Report your answer in nano meters.
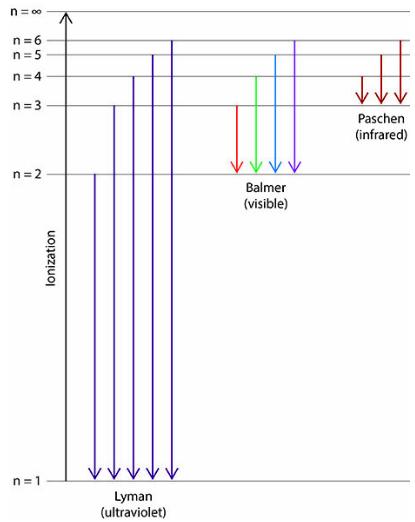
n= 00 n=6 n= 5 n=4 n= 3 Paschen (infrared) n= 2 Balmer (visible) n=1 Lyman (ultraviolet) lonization
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
a Energy of the radiation required to ionized electron from the grou...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


