Question
A chemical engineer is studying the rate of this reaction. 2H 3 PO 4 (aq) ? P 2 O 5 (aq) + 3H 2 O(aq)
A chemical engineer is studying the rate of this reaction.
2H 3 PO 4 (aq) ? P 2 O 5 (aq) + 3H 2 O(aq)
He fills a reaction vessel with H 3 PO 4 and measures its concentration as the reaction proceeds. Here?s graph of his data:
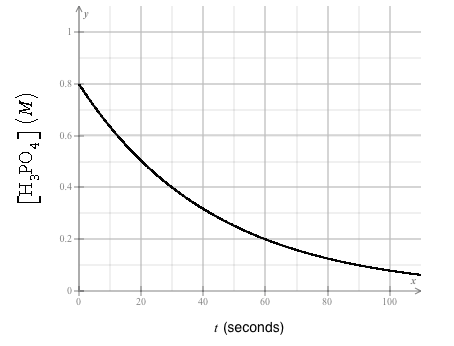
Use this graph answer the following questions:
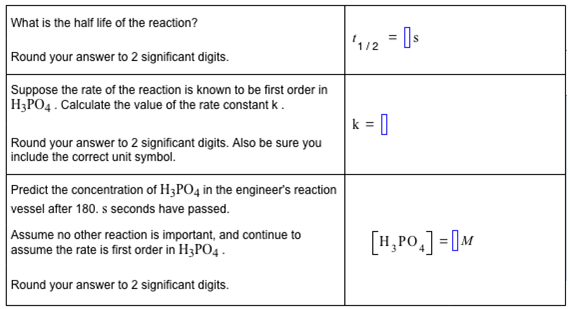
[HPO] (M) 0.8 0.6 0.4 0.2+ 0+ 20 40 60 t (seconds) 888.8 80 100 What is the half life of the reaction? Round your answer to 2 significant digits. Suppose the rate of the reaction is known to be first order in H3PO4. Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure you include the correct unit symbol. Predict the concentration of H3PO4 in the engineer's reaction vessel after 180. s seconds have passed. Assume no other reaction is important, and continue to assume the rate is first order in H3PO4. Round your answer to 2 significant digits. 1/2 = s |k=|| [HPO] = M
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
1 He fills a reaction vessel with H 3 PO 4 and measures its concentration as the re...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics For Engineers And Scientists
Authors: William Navidi
3rd Edition
73376345, 978-0077417581, 77417585, 73376337, 978-0073376332
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



