Question
A chemistry graduate student is studying the rate of this reaction: He fills a reaction vessel with H 3 PO 4 and measures its concentration
A chemistry graduate student is studying the rate of this reaction:
He fills a reaction vessel with H 3 PO 4 and measures its concentration as the reaction proceeds:
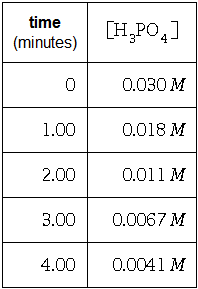
Use the data to answer the following questions.

time (minutes) 0 1.00 2.00 3.00 4.00 [HPO4] 0.030 M 0.018 M 0.011 M 0.0067 M 0.0041 M Write the rate law for this reaction. Calculate the value of the rate constant k Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. rate = 0 k = 0
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
One method to determine the rate law is hit and trial method For given data Use Different rate ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



