Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Assign formal charges to the atoms in the following structures. Which of the two do you think is the more important contributor to the resonance
Assign formal charges to the atoms in the following structures. Which of the two do you think is the more important contributor to the resonance hybrid.
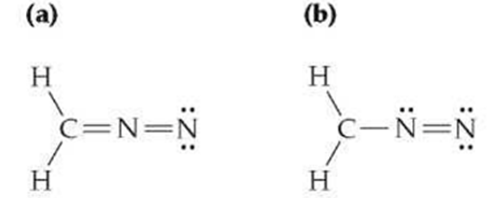
(a) H H C=N=N (b) H H C-N=N
Step by Step Solution
★★★★★
3.46 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Formal charges of the individual atoms can be calcu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6095d365b3c23_26295.pdf
180 KBs PDF File
6095d365b3c23_26295.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


