Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the linear density of ions along the [111] direction UO2, which has the CaF2 structure (Figure 1). Figure 1: Fluorite (CaF2) unit cell showing
Calculate the linear density of ions along the [111] direction UO2, which has the CaF2 structure (Figure 1).
Figure 1:
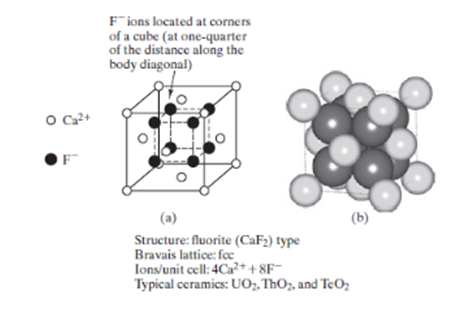
Fluorite (CaF2) unit cell showing (a) ion positions and (b) full-size ions. [Part (b) courtesy of Accelrys, Inc.]
O Ca+ F Fions located at corners of a cube (at one-quarter of the distance along the body diagonal) (b) (a) Structure: fluorite (CaF) type Bravais lattice: fcc Ions/unit cell: 4Ca++8F- Typical ceramics: UO, ThO, and TeO
Step by Step Solution
★★★★★
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
The structure of UO 2 unit cell is shown below The 111 direction line ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
609a4ca0166d5_30467.pdf
180 KBs PDF File
609a4ca0166d5_30467.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


