Question
Compare the solubility of nickel (II) carbonate in each of the following aqueous solutions: 0.10 M Ni(CH3COO) 0.10 M KCO3 0.10 M KNO3 0.10 M
Compare the solubility of nickel (II) carbonate in each of the following aqueous solutions: 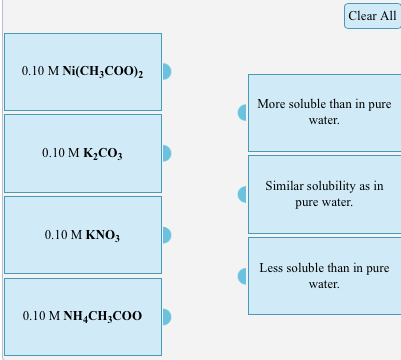
0.10 M Ni(CH3COO) 0.10 M KCO3 0.10 M KNO3 0.10 M NHCH3COO Clear All More soluble than in pure water. Similar solubility as in pure water. Less soluble than in pure water.
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Ni2CO3s Ni2aq CO32aq When we add solute having comm...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry A Short Course
Authors: Harold Hart, Christopher M. Hadad, Leslie E. Craine, David J. Hart
13th edition
1111425566, 978-1111425562
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



