Question
Complete the mechanism for the formation of the major species at equilibrium for the reaction of butanal in methanol solvent and catalytic aqueous acid. Make
Complete the mechanism for the formation of the major species at equilibrium for the reaction of butanal in methanol solvent and catalytic aqueous acid. Make sure to include any missing atoms, bonds, charges, nonbonding electrons, and curved arrows. Then classify the final product below.
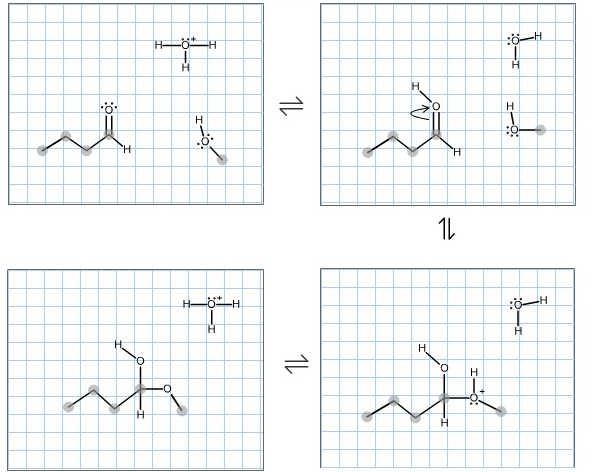
Select the choice that best describes the final structure.
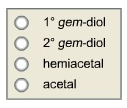
0 H -0 H H * H8H H 1L 1L H "H H 1L H H FO 0-H H H :0 28-4 H oooo 1 gem-diol gem-diol 2 hemiacetal O acetal
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
A 8 i H 4 H8H sti H 12 14 Select the choice that best describes the final ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



