Question
Complete the mechanism for the formation of the major species at equilibrium for the reaction of 2- methylpropanal in water and catalytic aqueous acid. Make
Complete the mechanism for the formation of the major species at equilibrium for the reaction of 2- methylpropanal in water and catalytic aqueous acid. Make sure to include any missing atoms, bonds, charges, non-bonding electrons and curved arrows. Then classify the final product below. 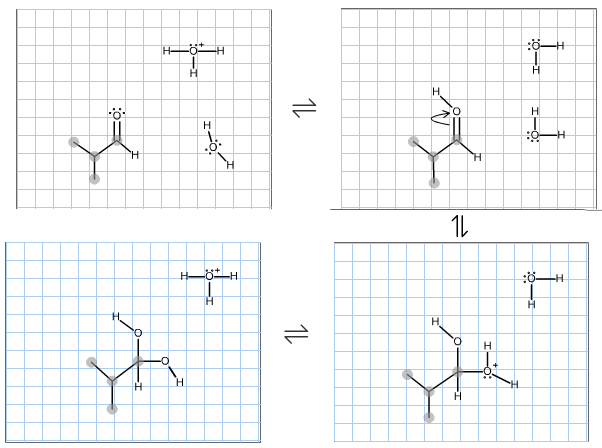
Select the choice that best describes the final structure.
A. 1? gem-diol
B. 2? gem-diol
C. Hemiacetal
D. Acetal
1.1 -1 H0H I SI < I -I :: 11. :8-H H__H H I I' I % O. I I
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
A Reaction of 2 methylpropanal in water and catal...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



