Question
In a certain chemical plant, a reversible fluid-phase isomerization A?B is carried out over a solid catalyst in a tubular packed-bed reactor. If the reaction
In a certain chemical plant, a reversible fluid-phase isomerization A?B is carried out over a solid catalyst in a tubular packed-bed reactor. If the reaction is so rapid that mass transfer between the catalyst surface and the bulk fluid is rate-limiting, show that the kinetics are described in terms of the bulk concentrations C A and C B by
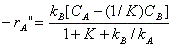
where -r " A = moles of A reacting per unit area catalyst per time k A , k B = mass transfer coefficients for A and B K = reaction equilibrium constant
It is desired to double the capacity of the existing plant by processing twice the feed of reactant A while maintaining the same fractional conversion of A to B in the reactor. How much larger a reactor, in terms of catalyst weight, would be required if all other operating variables are held constant? You may use the Thoenes-Kramaers correlation for mass transfer coefficients in a packed bed. Describe the effects of the flow rate, temperature, and particle size on conversion.
-TA"= _k[CA-(1/K)C] 1+ K + kg/kA B
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
A Mass transfer packed bed reversible reaction At high Temperature weak absorpt...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6096f99d8d751_27372.pdf
180 KBs PDF File
6096f99d8d751_27372.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


