Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Lithium Flouride, LiF, has the same crystal structure as NaCl and therefore has essentially the same Madelung constant ?. Its ionic cohesive energy is -10.5
Lithium Flouride, LiF, has the same crystal structure as NaCl and therefore has essentially the same Madelung constant ?. Its ionic cohesive energy is -10.5 eV and the value of n in the equation is 6.25, Find the equilibrium ionic separation in LiF.
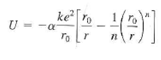
ke ke U=-a ro To r (0)1 n
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Its given that LiF has the same structure as NaCl ie ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6096c20cb895c_27169.pdf
180 KBs PDF File
6096c20cb895c_27169.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


