Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Oxidation of gaseous CIF by F 2 yields liquid CIF 3 , and important flouring agent. Use the following thermochemical equation to calculate ?H? rxn
Oxidation of gaseous CIF by F 2 yields liquid CIF 3 , and important flouring agent. Use the following thermochemical equation to calculate ?H? rxn for the production of CIF 3
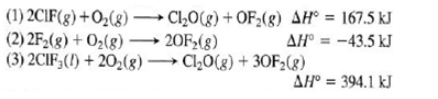
(1) 2CIF(g) + O(g) ClO(g) + OF(g) AH = 167.5 kJ (2) 2F(g) + O(g) 20F(g) AH = -43.5 kJ (3) 2CIF, () +20(g) ClO(g) + 30F(g) - AH = 394.1 kJ
Step by Step Solution
★★★★★
3.39 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
The chemical equation target equation for the formatio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6096d774e4a97_27246.pdf
180 KBs PDF File
6096d774e4a97_27246.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


