Research is being carried out on cellulose as a source of chemicals for the production of fibers, coatings, and plastics. Cellulose consists of long chains
Research is being carried out on cellulose as a source of chemicals for the production of fibers, coatings, and plastics. Cellulose consists of long chains of glucose molecules (C6H12O6), so for the purposes of modeling the reaction we can consider the conversion of glucose to formaldehyde (H2CO). Calculate the heat of reaction for the conversion of 1 mole of glucose into formaldehyde, given the following thermochemical data:
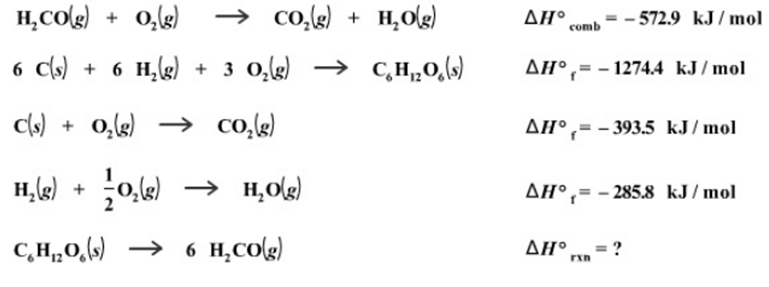
HCO(g) + O(g) co(g) + HO(g) 6 C(s) + 6 H(g) + 3 O(g) +3 0(g) CHO(s) C(s) + O(g) CO(g) H(g) + O(g) HO(g) C.HO(s) 6 HCO(g) comb AH, -1274.4 kJ/mol AH= -393.5 kJ/mol AH -572.9 kJ/mol AH= -285.8 kJ/mol rxn = ?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Heat of reaction is the difference in t...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


