Question
The following ten equations are purported to be fundamental equations of various thermodynamic systems. However, five are inconsistent with one or more of postulates II,
The following ten equations are purported to be fundamental equations of various thermodynamic systems. However, five are inconsistent with one or more of postulates II, III, and IV and consequently arc not physically acceptable. In each case qualitatively sketch the fundamental relationship between S and U (with N and Y constant). Find the five equations that are not physically permissible and indicate the postulates violated by each.
The quantities v o , ?, and R are positive constants, and in all cases in which fractional exponents appear only the real positive root is to be taken.
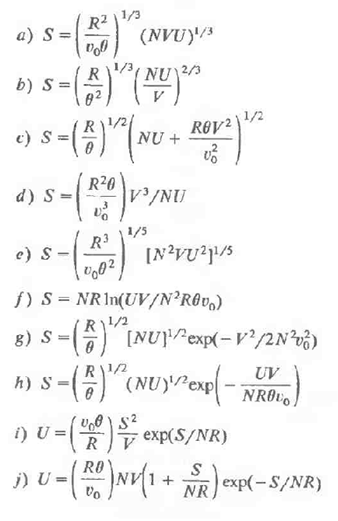
R a) c) S = (R) (NVU)/3 (NVUY/x R 2/3 b) S = (^)^^ (NU) S= e) S-(A)^ (NU + RV/ R c) = ROV2 d) S = (R0) 1/NU - 1/5 R (1802) (0111/5 e) S f) S = NRIn(UV/NR0vo) 8) S (8)[NU/_exp(-V/2N23) A) S-(=) (NU)/"exp(- NRI) R UV h) 2 1) U = (1/) = 7 exp(S/NR) RV S 1) U = (RO)NV(1 + R) exp(-S/NR) NR
Step by Step Solution
3.33 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
If U be the total internal energy H be the enthalpy F ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60959014a2730_26002.pdf
180 KBs PDF File
60959014a2730_26002.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


