Question
The ideal gas law provides one way to estimate the pressure exerted by a gas in a container. The law is P = nRT/V More
The ideal gas law provides one way to estimate the pressure exerted by a gas in a container. The law is
P = nRT/V
More accurate estimates can be made with the van der Waals equation:
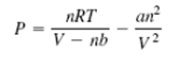
where the term nb is a correction for the volume of the molecules, and the term an 2 /V 2 is a correction for molecular attractions for molecular attractions. The values of a and b depend on the type of gas. The gas constant is R, the absolute temperature is T, the gas volume is V, and the number of gas molecules is indicated by n. If n = 1 mol of an ideal gas were confined to a volume of V = 22.41 L at 0?C (273.2 K), it would exert a pressure of 1 atmosphere. In these units, R = 0.08206.
For chlorine (Cl 2 ), a = 6.49 and b = 0.0562. Compare the pressure estimates given by the ideal gas law and the van der Waals equation for 1 mol of Cl 2 in 22.41 L at 273.2 K. What is the main cause of the difference in the two pressure estimates: the molecular volume or the molecular attractions?
P= nRT an V V-nb
Step by Step Solution
3.40 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The session is The ideal gas law predicts ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60958e156ac1c_25993.pdf
180 KBs PDF File
60958e156ac1c_25993.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


