Question
The position of the OH resonance of phenol varies with concentration is solution, as the following table shows. On the other hand, the hydroxyl proton
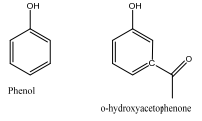
The position of the OH resonance of phenol varies with concentration is solution, as the following table shows. On the other hand, the hydroxyl proton of o-hydroxyacetophenone appears at 12.05 ppm and does not show any great shift upon dilution. Explain.
Concentration w/c in CCI 4 (ppm)
100%.................................................................7.45
20%...................................................................6.75
10%...................................................................6.45
5%.....................................................................5.95
2%.....................................................................4.88
1%.....................................................................4.37
OH Phenol OH o-hydroxyacetophenone
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
The chemical shift value of OH is strongly affected by its hydrogen bonding abili...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60952d47cf34d_25620.pdf
180 KBs PDF File
60952d47cf34d_25620.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


