Question
Consider the following system at equilibrium where AH 92.7 kJ, and K. =1.80x10 at 298 K NH,HS()NH3(g) + H,S(g) When some moles of NH
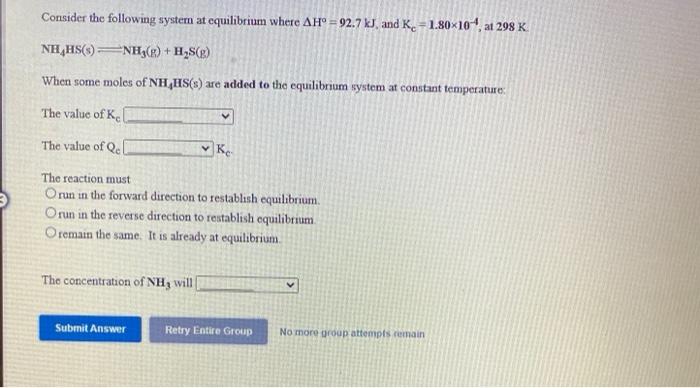
Consider the following system at equilibrium where AH 92.7 kJ, and K. =1.80x10 at 298 K NH,HS()NH3(g) + H,S(g) When some moles of NH HS(s) are added to the equilibrium system at constant temperature: The value of K. The value of Qe Ke The reaction must Orun in the forward direction to restablsh equilibrium. Orun in the reverse direction to restablish equilibrium O remain the same. It is already at equilibrium. The concentration of NH3 wil Submit Answer Retry Entire Group No more group attempts remain
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
The equilibrium reaction is NH4HSs NH3g H2Sg at equilibriu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



