Question: According to the Law of Conservation of Mass, matter is not created or destroyed in a chemical reaction. In this experiment, you will explore

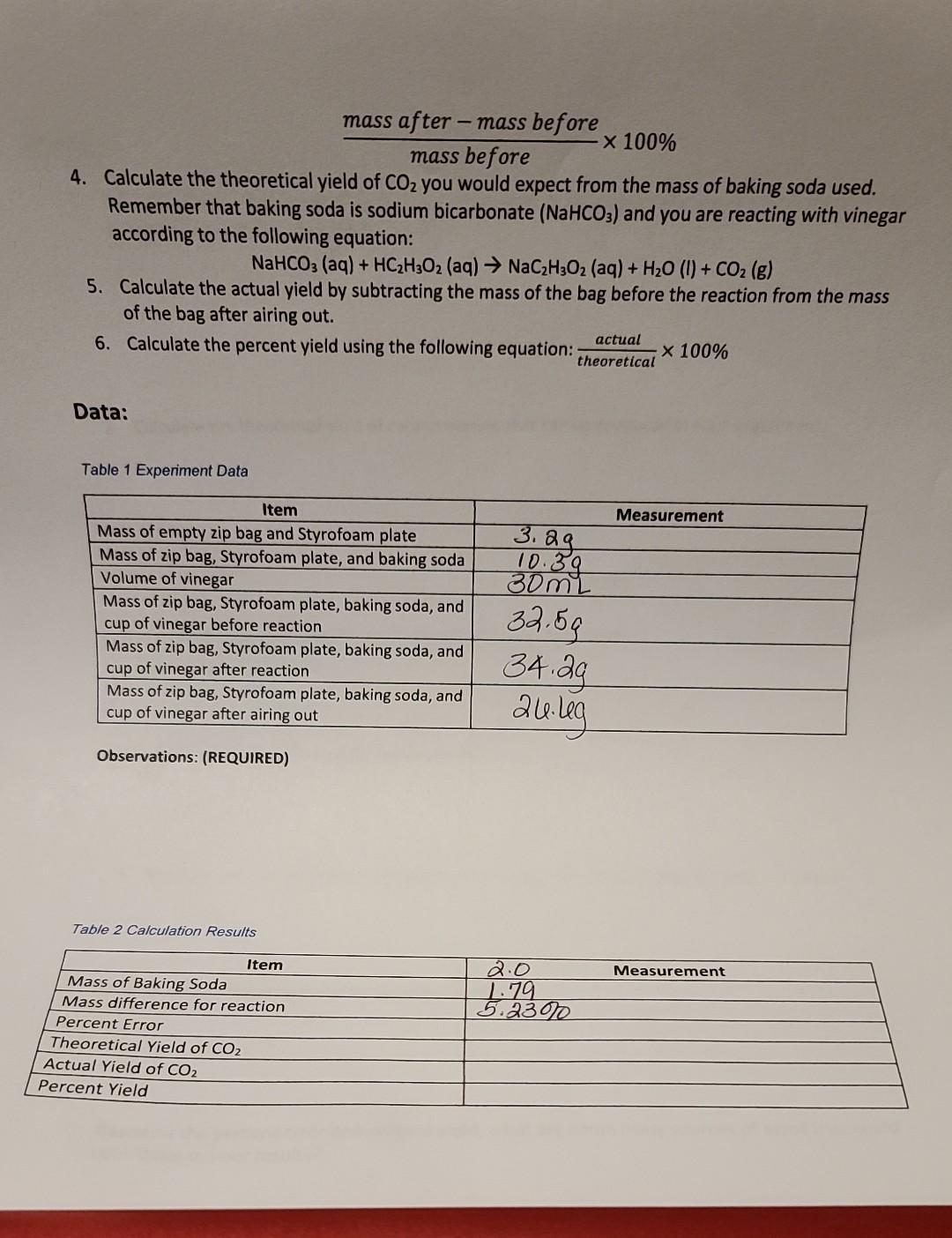

According to the Law of Conservation of Mass, matter is not created or destroyed in a chemical reaction. In this experiment, you will explore this concept by keeping a reaction contained in a sealed container. Make sure that you are careful with each measurement and that your container is sealed to ensure maximum accuracy. Equipment: 1. Styrofoam plate Baking soda (sodium bicarbonate [NaHCO3]) Vinegar (acetic acid [HCH30]) Two small paper cups Graduated cylinder Scale 8. Procedure: 1. 7. Place your plastic zip bag and Styrofoam plate on the scale and record the mass below. Be sure to record all digits. 2. Add approximately 2 g of baking soda into the bag and weigh the baking soda and bag and Styrofoam plate together. Record the mass below, with all digits. 3. Measure 30.0 mL of vinegar in your graduated cylinder and pour into one of the small cups. Record the actual volume used below. 4. Make sure that the cup is dry on the outside. Carefully place the cup into the zip bag with the vinegar, making sure that none of the vinegar spills on the baking soda. Carefully seal the bag, zero the balance, and place the bag and Styrofoam plate on the scale to weigh it. Be careful not to spill the vinegar on the baking soda during this process. Record the mass below, with all digits. Once you have recorded the mass of the bag, keep the bag sealed while you carefully invert the cup. Allow the vinegar to mix with the baking soda thoroughly. (REQUIRED) Record any observations of what occurs, taking special note of the temperature. Once the reaction is complete, keep the bag sealed, weigh it on the Styrofoam plate, and record the mass below, with all digits. Open the bag and let it air out for at least 30 minutes. After at least 30 minutes, weigh the bag with contents and the Styrofoam plate and record the mass below. 9. Once you have recorded all of the data, pour the liquid out of the bag into the sink and dispose of any disposable components in the trash. Quart size plastic zip bag 5. 6. Calculations Find the mass of baking soda by subtracting the mass of the zip bag and Styrofoam plate from the mass of zip bag, Styrofoam plate, and baking soda 2. Calculate the mass difference by subtracting the mass before the reaction from the mass after the reaction 3. Calculate the percent error by dividing the mass difference calculated in step 2 by the mass before the reaction started and multiplying by 100%: mass after-mass before x 100% mass before 4. Calculate the theoretical yield of CO you would expect from the mass of baking soda used. Remember that baking soda is sodium bicarbonate (NaHCO3) and you are reacting with vinegar according to the following equation: NaHCO3(aq) + HC2H3O2 (aq) NaCH3O2 (aq) + HO (1) + CO(g) 5. Calculate the actual yield by subtracting the mass of the bag before the reaction from the mass of the bag after airing out. 6. Calculate the percent yield using the following equation: actual theoretical Data: Table 1 Experiment Data Item Mass of empty zip bag and Styrofoam plate Mass of zip bag, Styrofoam plate, and baking soda Volume of vinegar Mass of zip bag, Styrofoam plate, baking soda, and cup of vinegar before reaction Mass of zip bag, Styrofoam plate, baking soda, and cup of vinegar after reaction Mass of zip bag, Styrofoam plate, baking soda, and cup of vinegar after airing out Observations: (REQUIRED) Table 2 Calculation Results Item Mass of Baking Soda Mass difference for reaction Percent Error Theoretical Yield of CO Actual Yield of CO Percent Yield 3. 3. 2g. 10.39 30mL 32.59 34.29 24.leg 2.0 1.79 5.2300 x 100% Measurement Measurement Analysis: 1. Calculate the percent error between the initial mass of the bag before the reaction and the mass of the unopened bag after the reaction. 2. Calculate the theoretical yield of carbon dioxide that can be produced in your experiment. 3. Calculate the actual yield of carbon dioxide from your experiment. 4. Calculate the percent yield for your reaction. 5. Based on your percent error, does this experiment illustrate the Law of Conservation of Mass? 6. How close is the actual yield to the theoretical? 7. Based on the percent error and percent yield, what are some likely sources of error that could contribute to your results?
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
1 ride with enthroom ... View full answer

Get step-by-step solutions from verified subject matter experts


