Answered step by step
Verified Expert Solution
Question
1 Approved Answer
009 10.0 points Three liquids are at temperatures of 4C, 24C, and 29C, respectively. Equal masses of the first two liquids are mixed, and
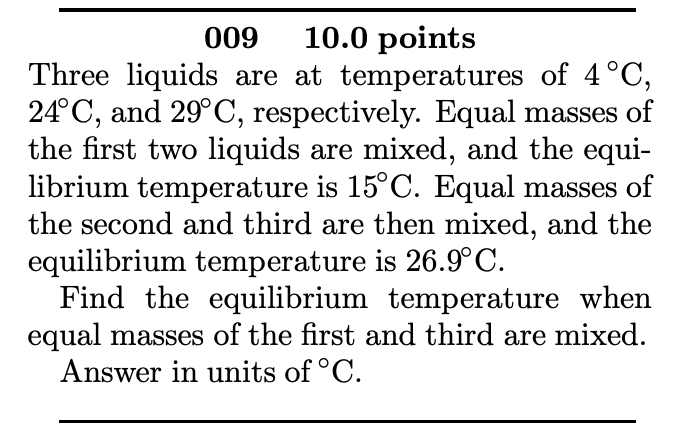
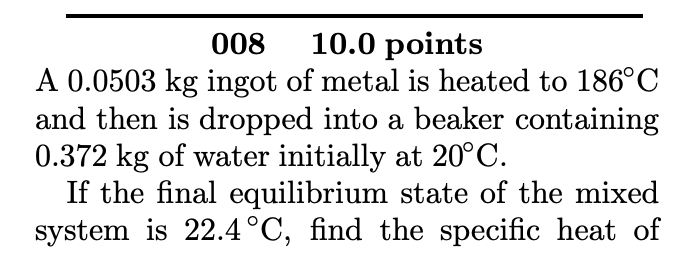
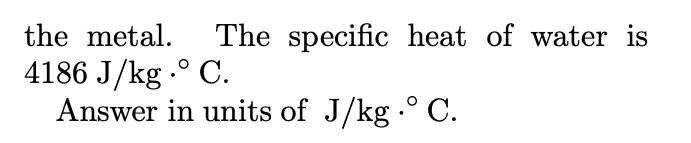
009 10.0 points Three liquids are at temperatures of 4C, 24C, and 29C, respectively. Equal masses of the first two liquids are mixed, and the equi- librium temperature is 15C. Equal masses of the second and third are then mixed, and the equilibrium temperature is 26.9C. Find the equilibrium temperature when equal masses of the first and third are mixed. Answer in units of C. 008 10.0 points A 0.0503 kg ingot of metal is heated to 186C and then is dropped into a beaker containing 0.372 kg of water initially at 20C. If the final equilibrium state of the mixed system is 22.4C, find the specific heat of the metal. The specific heat of water is 4186 J/kg. C. Answer in units of J/kg. C.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


