Answered step by step
Verified Expert Solution
Question
1 Approved Answer
0.24gCH_(4) are burned and all the heat added to a silver cup with 85gH_(2)O_((I)) inside. If the water was heated up 25C what mass in
0.24gCH_(4)are burned and all the heat added to a silver cup with
85gH_(2)O_((I))inside. If the water was heated up
25Cwhat mass in grams was the silver cup?\
S_(Ag)=0.233(J)/(g)K(Joules per gram per kelvin)\
dH_(rxn)CH_(4)burning
=-890kJ 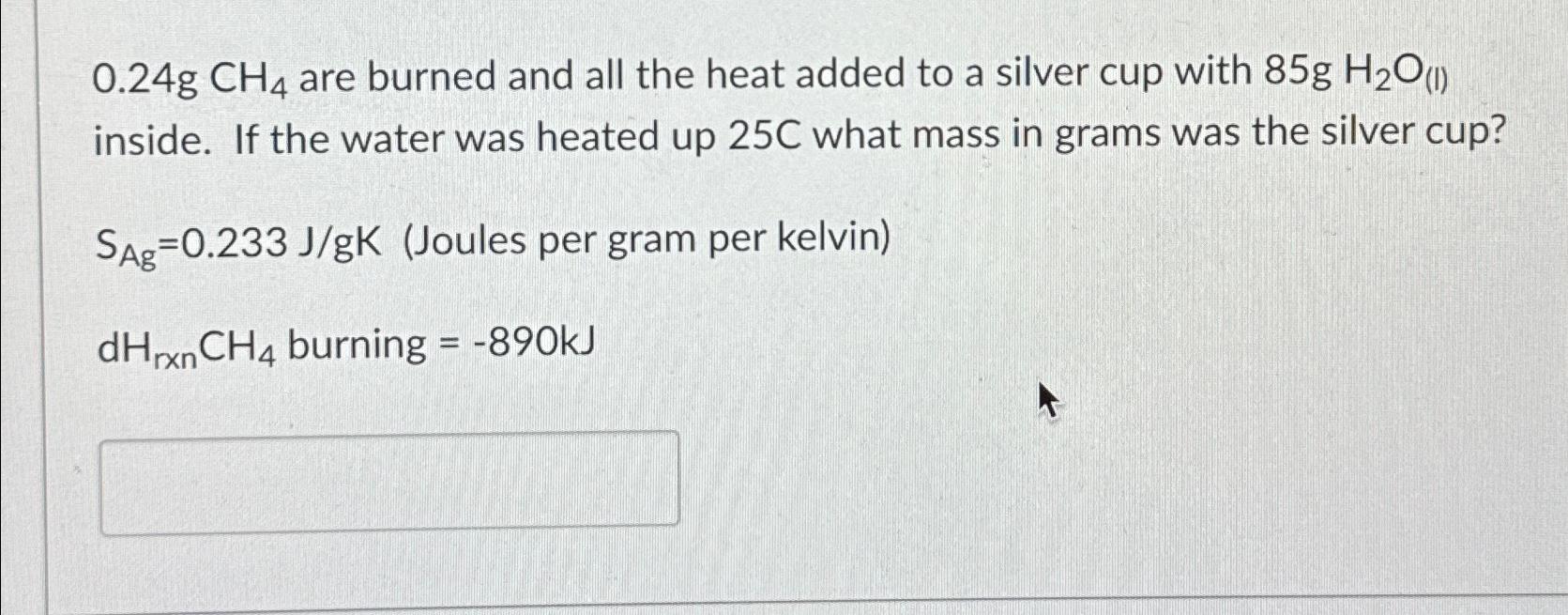
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


