Question
0.325 kg of neon (an inert, ideal gas) receives1139 kJ of energy transferred into the system as heat transfer during a process. Temperature measurements
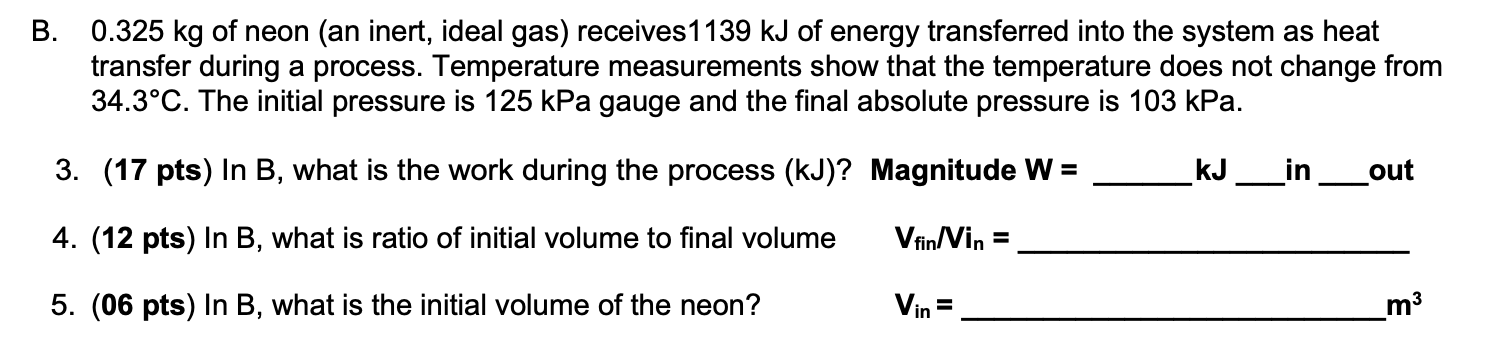
0.325 kg of neon (an inert, ideal gas) receives1139 kJ of energy transferred into the system as heat transfer during a process. Temperature measurements show that the temperature does not change from 34.3C. The initial pressure is 125 kPa gauge and the final absolute pressure is 103 kPa. 3. (17 pts) In B, what is the work during the process (kJ)? Magnitude W = 4. (12 pts) In B, what is ratio of initial volume to final volume Vfin/Vin = 5. (06 pts) In B, what is the initial volume of the neon? Vin= B. kJ in out m
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Solution 3 Work done W Since the temperature remains constant throughout the process isothermal the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Physics
Authors: Jearl Walker, Halliday Resnick
8th Extended edition
471758019, 978-0471758013
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



