Answered step by step
Verified Expert Solution
Question
1 Approved Answer
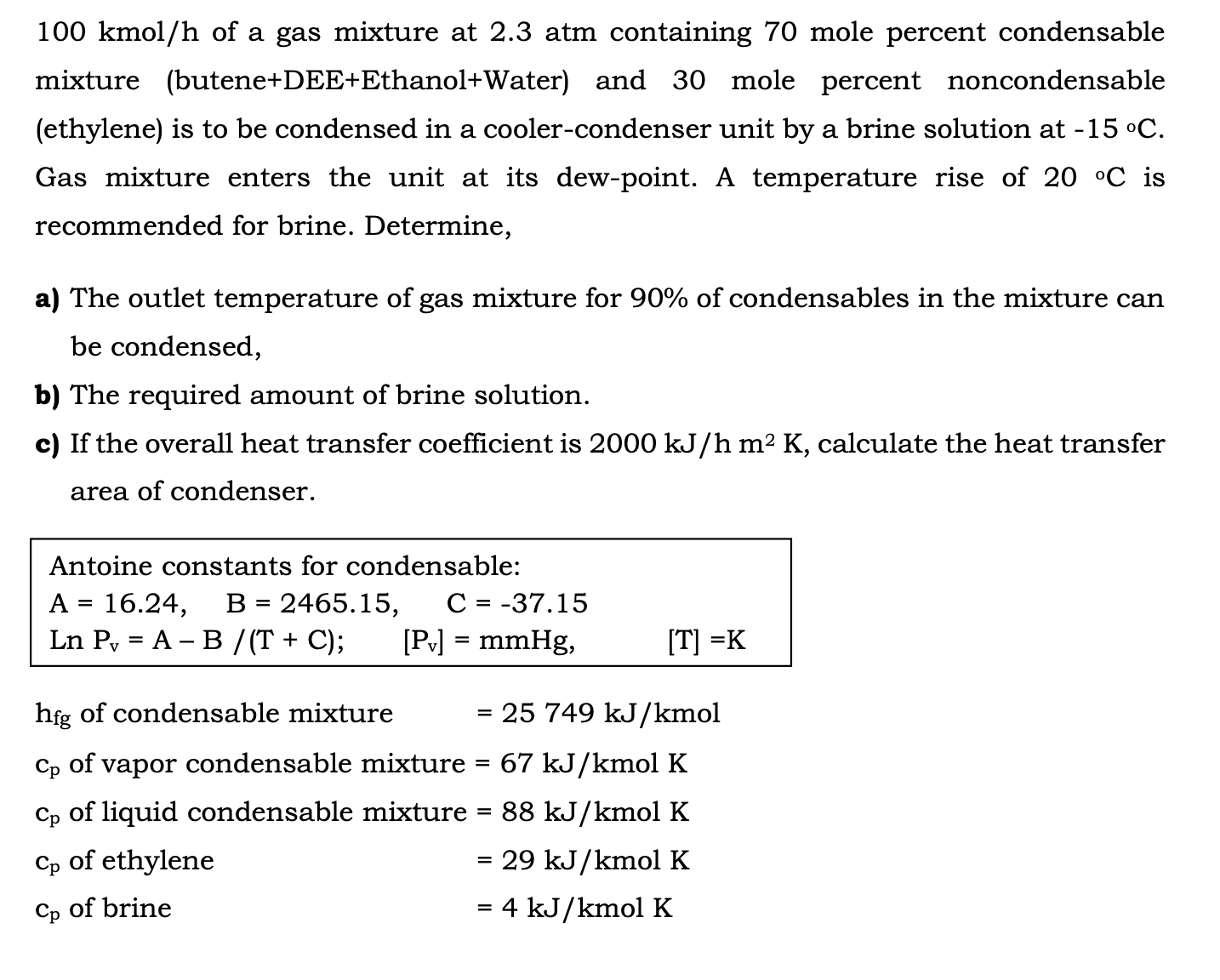
1 0 0 kmo l h of a gas mixture at 2 . 3 atm containing 7 0 mole percent condensable mixture ( butene +
kmo of a gas mixture at atm containing mole percent condensable
mixture buteneDEEEthanolWater and mole percent noncondensable
ethylene is to be condensed in a coolercondenser unit by a brine solution at
Gas mixture enters the unit at its dewpoint. A temperature rise of is
recommended for brine. Determine,
a The outlet temperature of gas mixture for of condensables in the mixture can
be condensed,
b The required amount of brine solution.
c If the overall heat transfer coefficient is calculate the heat transfer
area of condenser.
Antoine constants for condensable:
;
of condensable mixture mol
of vapor condensable mixture molK
of liquid condensable mixture molK
of ethylene molK
of brine molK
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


