Answered step by step
Verified Expert Solution
Question
1 Approved Answer
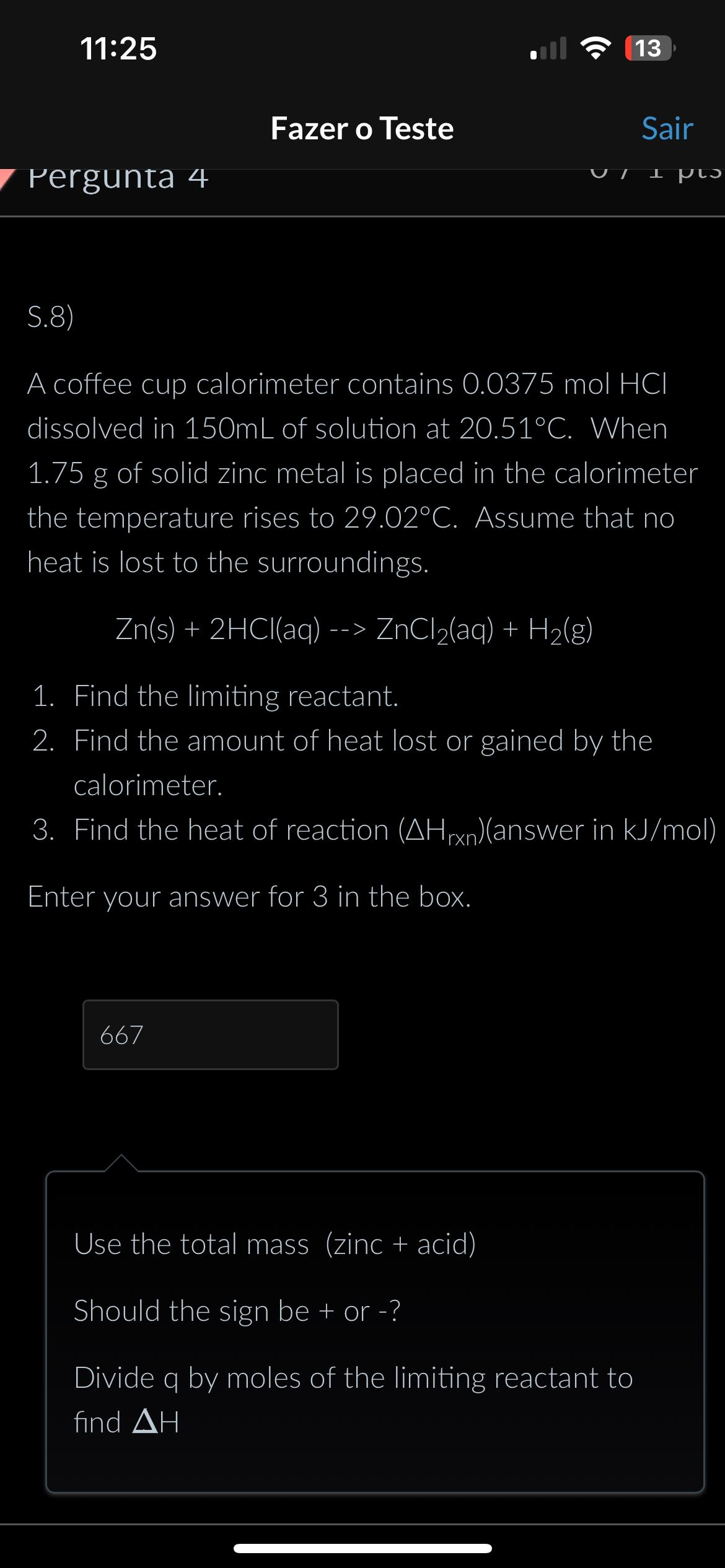
1 1 : 2 5 1 3 Fazer o Teste Sair Pergunta 4 S . 8 ) A coffee cup calorimeter contains 0 . 0
:
Fazer o Teste
Sair
Pergunta
S
A coffee cup calorimeter contains molHCl dissolved in of solution at When of solid zinc metal is placed in the calorimeter the temperature rises to Assume that no heat is lost to the surroundings.
Find the limiting reactant.
Find the amount of heat lost or gained by the calorimeter.
Find the heat of reaction answer in
Enter your answer for in the box.
Use the total mass zinc acid
Should the sign be or
Divide by moles of the limiting reactant to find
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


