Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (12 points) Consider the oligopeptide given below (written in the 3-letter code): Thr-Cys-Lys-Val-Tyr-Asn . Draw its full chemical structure as it would exist at
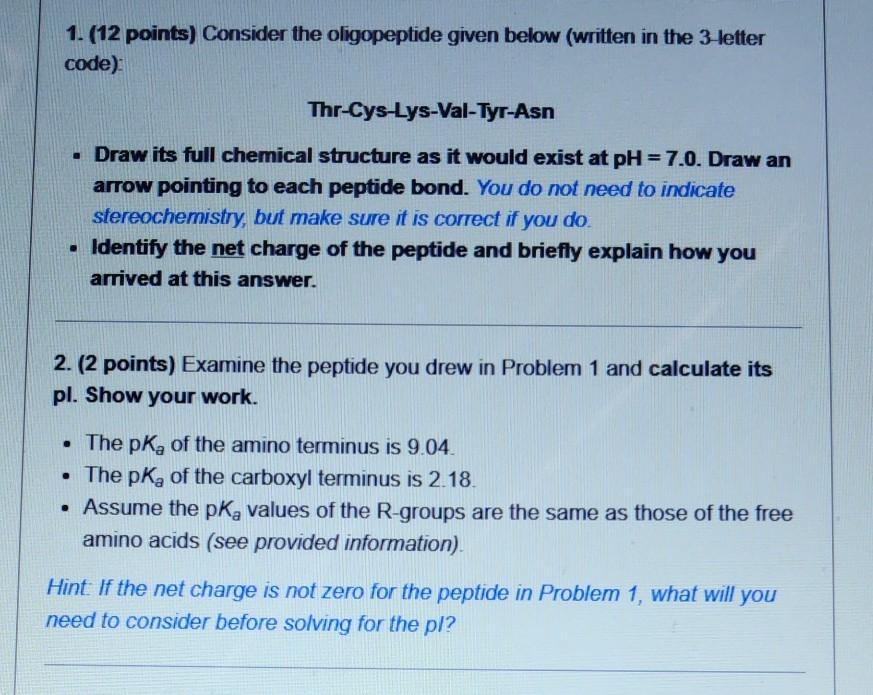
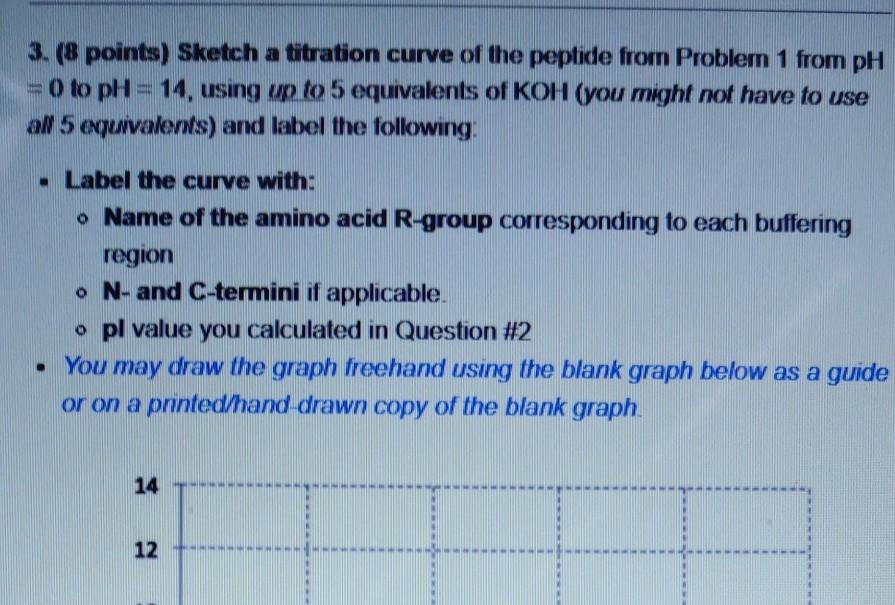
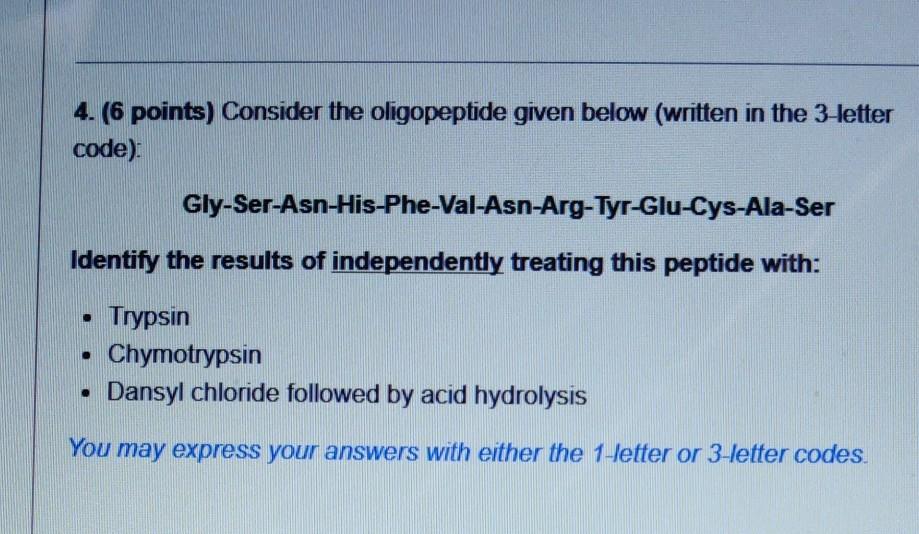
1. (12 points) Consider the oligopeptide given below (written in the 3-letter code): Thr-Cys-Lys-Val-Tyr-Asn . Draw its full chemical structure as it would exist at pH = 7.0. Draw an arrow pointing to each peptide bond. You do not need to indicate stereochemistry, but make sure it is correct if you do. Identify the net charge of the peptide and briefly explain how you arrived at this answer. 2. (2 points) Examine the peptide you drew in Problem 1 and calculate its pl. Show your work. The pk, of the amino terminus is 9.04. The pk, of the carboxyl terminus is 2.18. Assume the pk, values of the R-groups are the same as those of the free amino acids (see provided information) Hint If the net charge is not zero for the peptide in Problem 1, what will you need to consider before solving for the pl? 3. (8 points) Sketch a titration curve of the peptide from Problem 1 from PH = 0 to pH = 14, using up to 5 equivalents of KOH (you might not have to use al 5 euivalents) and label the following: Label the curve with: o Name of the amino acid R-group corresponding to each buffering region o N- and C-termini if applicable. o pl value you calculated in Question #2 You may draw the graph freehand using the blank graph below as a guide or on a pnnted/hand drawn copy of the blank graph. 14 12 4. (6 points) Consider the oligopeptide given below (written in the 3-letter code): Gly-Ser-Asn-His-Phe-Val-Asn-Arg-Tyr-Glu-Cys-Ala-Ser Identify the results of independently treating this peptide with: . Trypsin Chymotrypsin Dansyl chloride followed by acid hydrolysis . You may express your answers with either the 1-letter or 3-letter codes
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


