Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) (14 points) Complete the following chart. Name a. b. C. d. Isotopic notation 139 56 Ba Thorium-233 Atomic number (Z) 101 Atomic mass
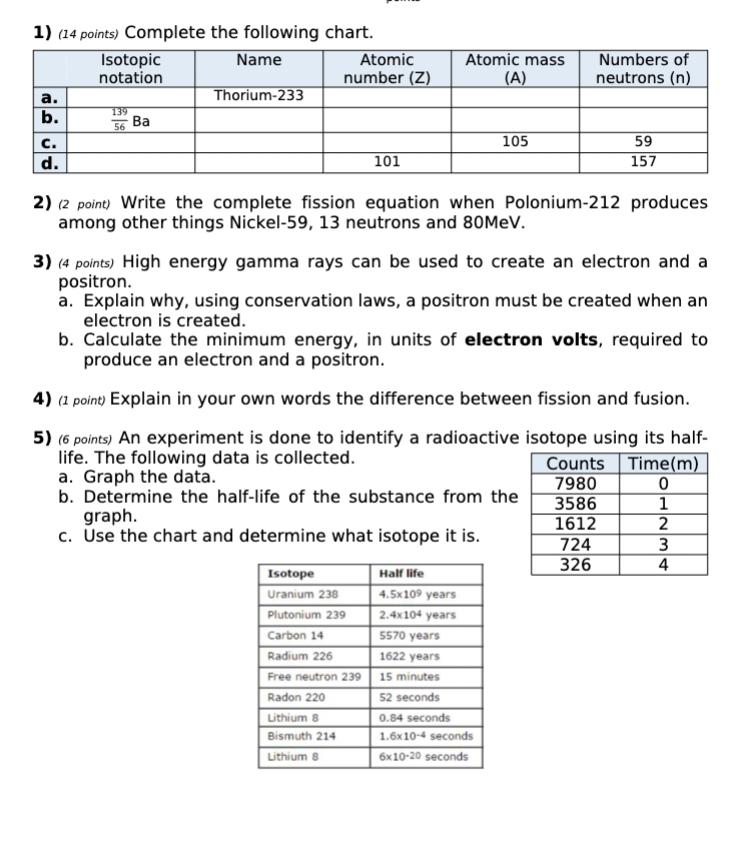
1) (14 points) Complete the following chart. Name a. b. C. d. Isotopic notation 139 56 Ba Thorium-233 Atomic number (Z) 101 Atomic mass (A) Isotope Uranium 238 Plutonium 239 2) (2 point) Write the complete fission equation when Polonium-212 produces among other things Nickel-59, 13 neutrons and 80MeV. graph. c. Use the chart and determine what isotope it is. Carbon 14 Radium 226 Free neutron 239 Radon 220 Lithium 8 Bismuth 214 Lithium 8 105 3) (4 points) High energy gamma rays can be used to create an electron and a positron. a. Explain why, using conservation laws, a positron must be created when an electron is created. b. Calculate the minimum energy, in units of electron volts, required to produce an electron and a positron. 4) (1 point) Explain in your own words the difference between fission and fusion. 5) (6 points) An experiment is done to identify a radioactive isotope using its half- life. The following data is collected. a. Graph the data. b. Determine the half-life of the substance from the Numbers of neutrons (n) Half life 4.5x109 years 2.4x104 years 5570 years 1622 years 15 minutes 52 seconds 0.84 seconds 1.6x10-4 seconds 6x10-20 seconds 59 157 724 326 Counts Time(m) 7980 0 3586 1 1612 2 3 4
Step by Step Solution
★★★★★
3.49 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1
Solutions the given isotope is radon 220 calculated value ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


