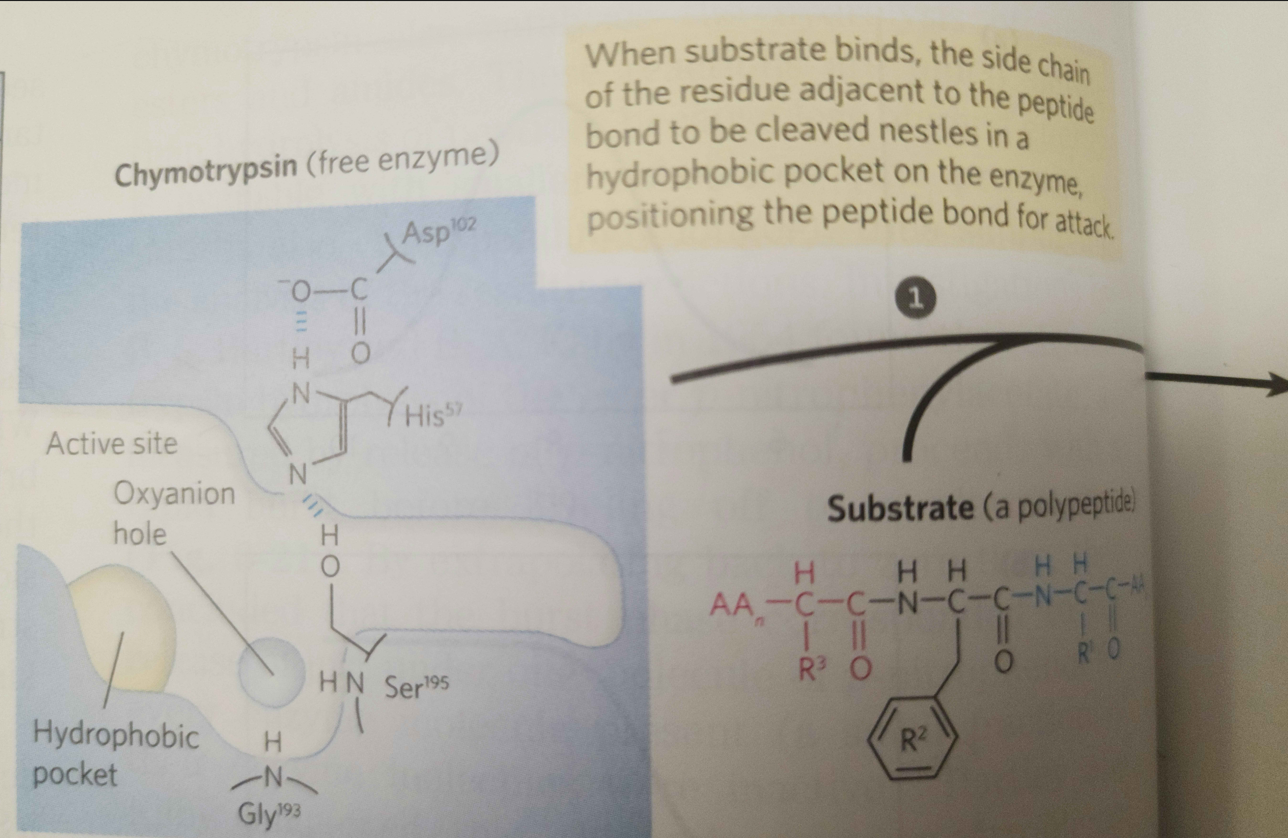
1.
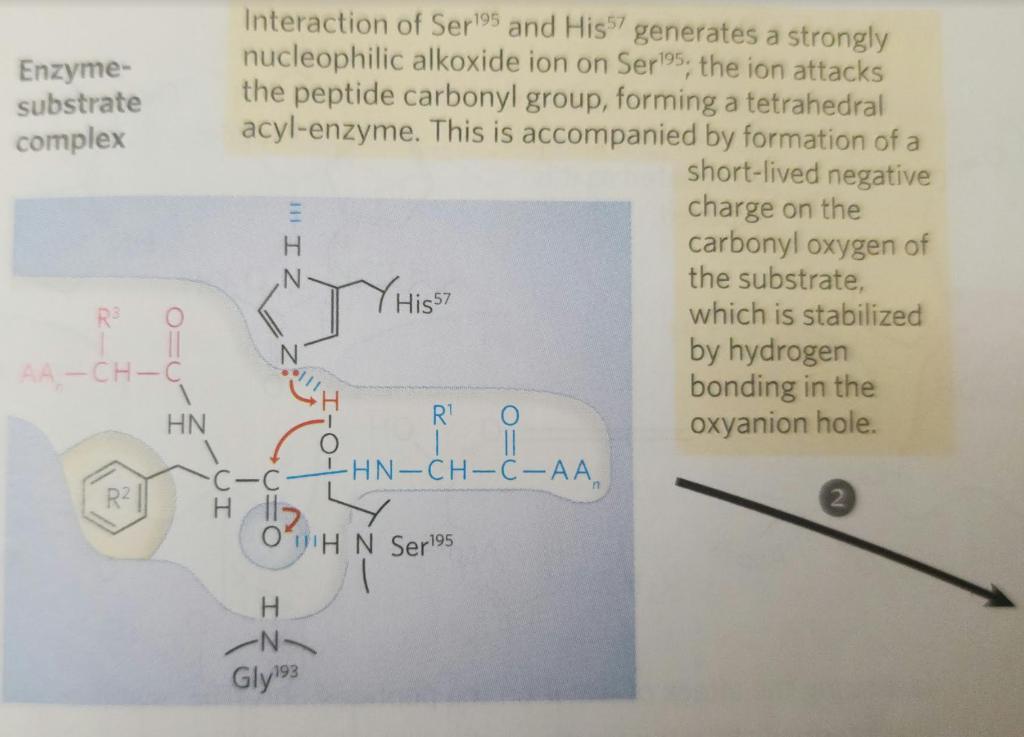
2.
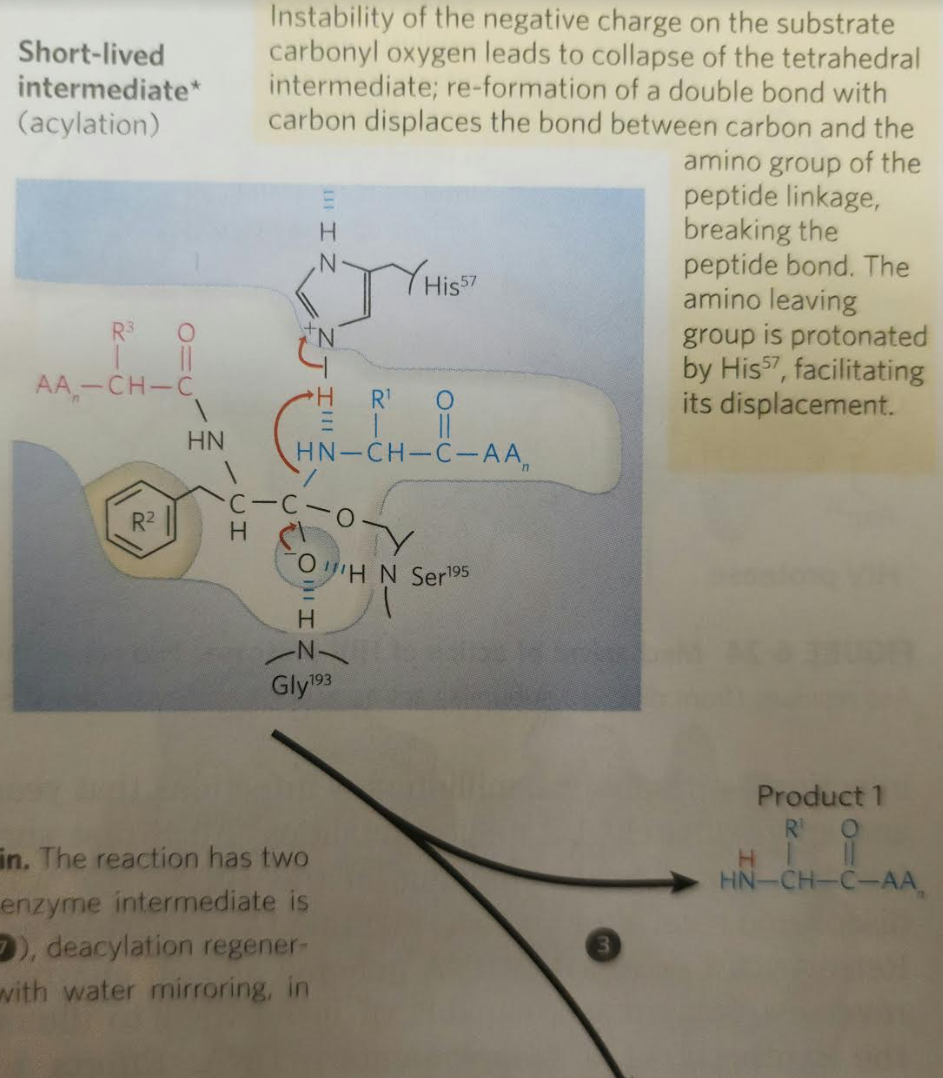
3.
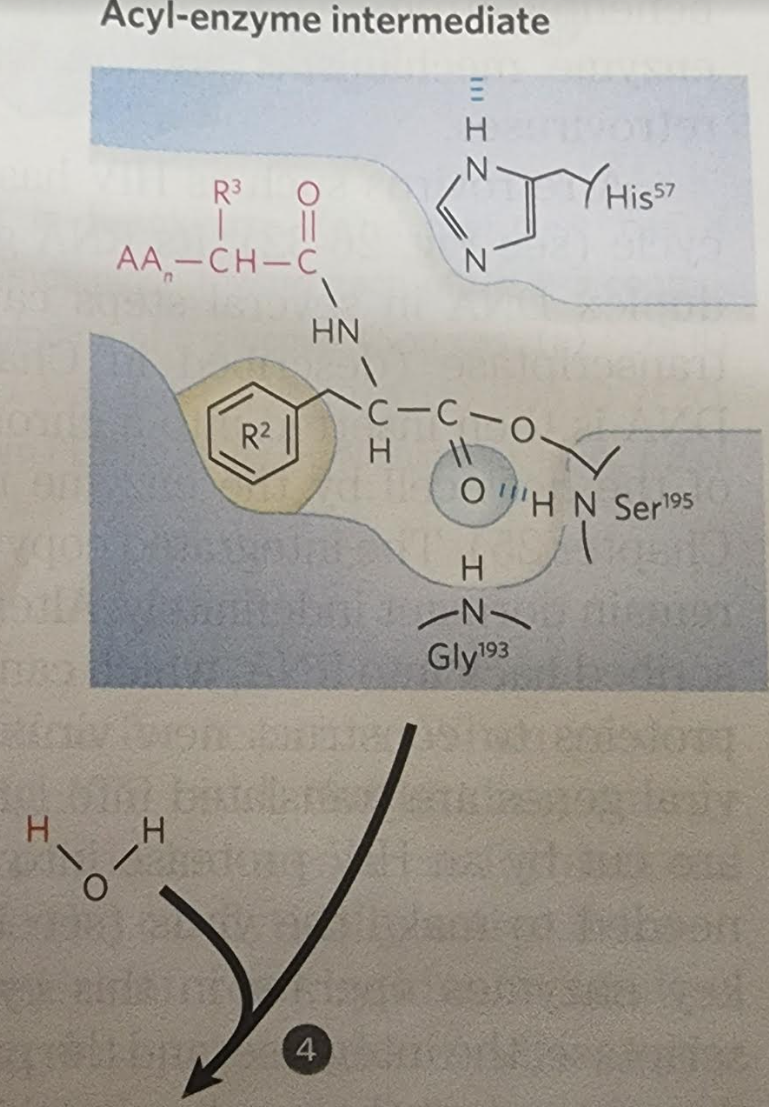
4.
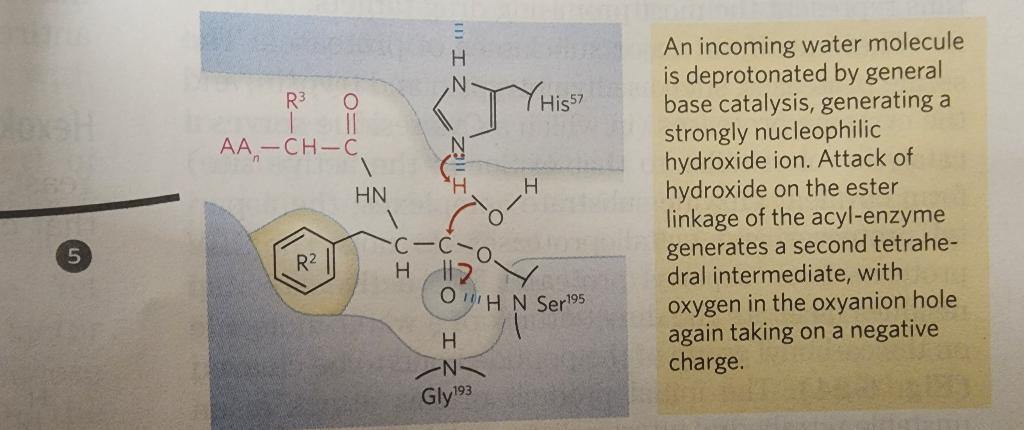
5.
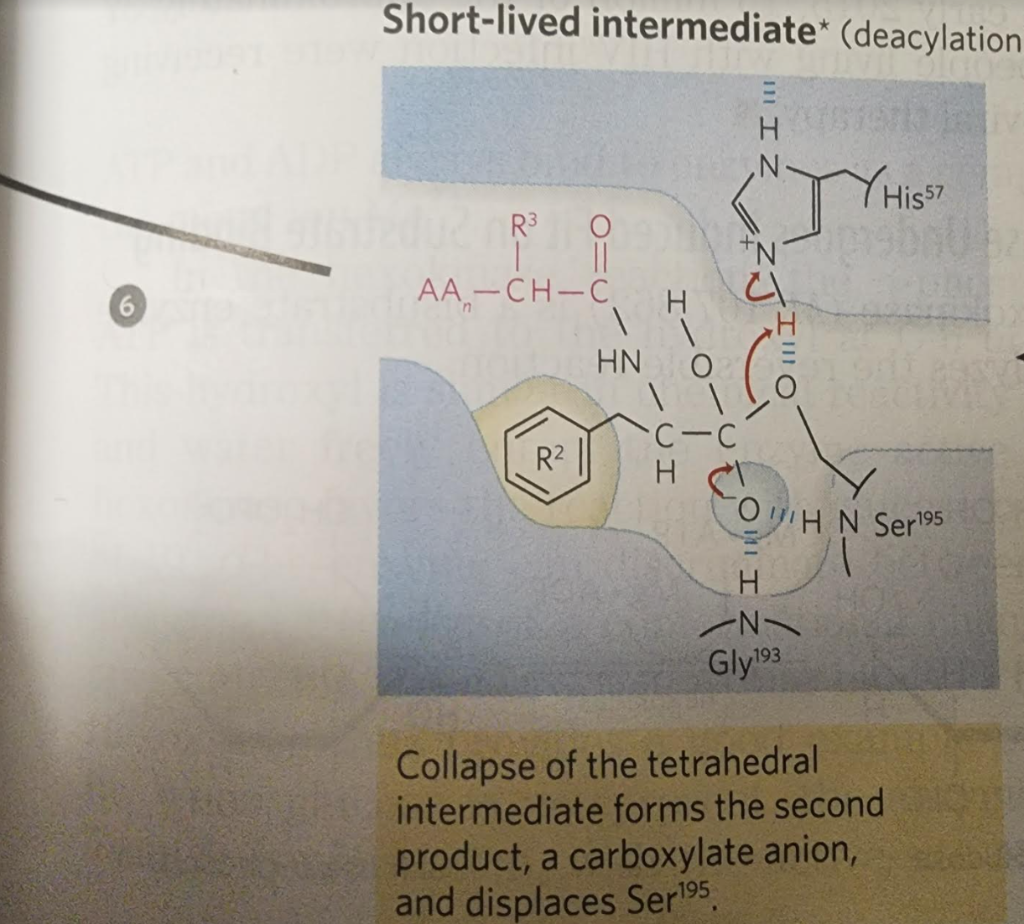
6.
7.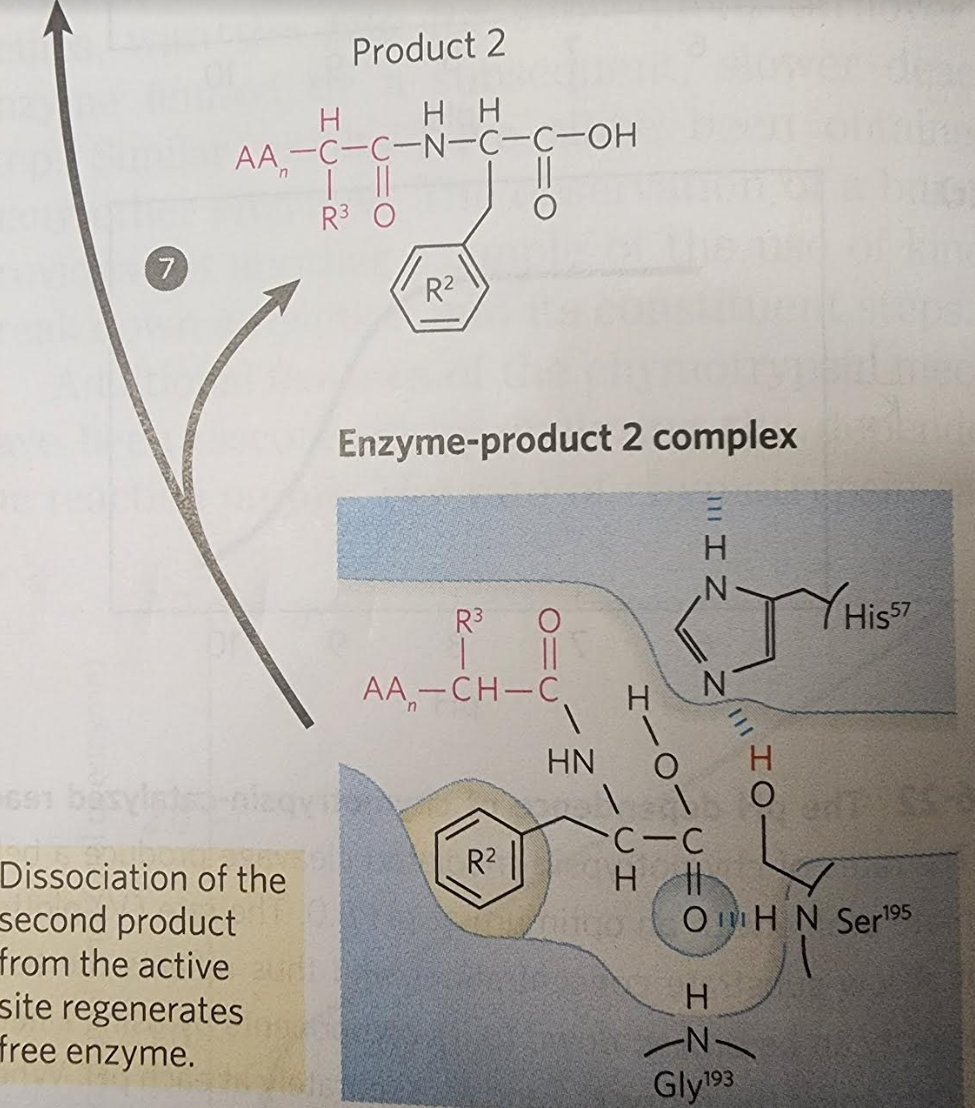 For each red arrow shown inside the picture (for the steps 1 through 7) label what type of chemistry it is: nucleophilic attack, base catalysis, acid catalysis, enzyme substrate complex, redox reaction etc.
For each red arrow shown inside the picture (for the steps 1 through 7) label what type of chemistry it is: nucleophilic attack, base catalysis, acid catalysis, enzyme substrate complex, redox reaction etc.
When substrate binds, the side chain of the residue adjacent to the peptide pond to be cleaved nestles in a hydrophobic pocket on the enzyme, bositioning the peptide bond for attack. Interaction of Ser195 and His57 generates a strongly nucleophilic alkoxide ion on Ser 195; the ion attacks the peptide carbonyl group, forming a tetrahedral acyl-enzyme. This is accompanied by formation of a Instability of the negative charge on the substrate Short-lived carbonyl oxygen leads to collapse of the tetrahedral intermediate intermediate; re-formation of a double bond with (acylation) carbon displaces the bond between carbon and the amino group of the peptide linkage, breaking the peptide bond. The amino leaving group is protonated by His57, facilitating its displacement. in. The reaction has two enzyme intermediate is ), deacylation regener- 3 with water mirroring, in Acyl-enzyme intermediate An incoming water molecule is deprotonated by general base catalysis, generating a strongly nucleophilic hydroxide ion. Attack of hydroxide on the ester linkage of the acyl-enzyme generates a second tetrahedral intermediate, with oxygen in the oxyanion hole again taking on a negative charge. Short-lived intermediate (deacylation Collapse of the tetrahedral intermediate forms the second product, a carboxylate anion, and displaces Ser 195. Product 2 Enzyme-product 2 complex Dissociation of the second product from the active site regenerates free enzyme. When substrate binds, the side chain of the residue adjacent to the peptide pond to be cleaved nestles in a hydrophobic pocket on the enzyme, bositioning the peptide bond for attack. Interaction of Ser195 and His57 generates a strongly nucleophilic alkoxide ion on Ser 195; the ion attacks the peptide carbonyl group, forming a tetrahedral acyl-enzyme. This is accompanied by formation of a Instability of the negative charge on the substrate Short-lived carbonyl oxygen leads to collapse of the tetrahedral intermediate intermediate; re-formation of a double bond with (acylation) carbon displaces the bond between carbon and the amino group of the peptide linkage, breaking the peptide bond. The amino leaving group is protonated by His57, facilitating its displacement. in. The reaction has two enzyme intermediate is ), deacylation regener- 3 with water mirroring, in Acyl-enzyme intermediate An incoming water molecule is deprotonated by general base catalysis, generating a strongly nucleophilic hydroxide ion. Attack of hydroxide on the ester linkage of the acyl-enzyme generates a second tetrahedral intermediate, with oxygen in the oxyanion hole again taking on a negative charge. Short-lived intermediate (deacylation Collapse of the tetrahedral intermediate forms the second product, a carboxylate anion, and displaces Ser 195. Product 2 Enzyme-product 2 complex Dissociation of the second product from the active site regenerates free enzyme






 For each red arrow shown inside the picture (for the steps 1 through 7) label what type of chemistry it is: nucleophilic attack, base catalysis, acid catalysis, enzyme substrate complex, redox reaction etc.
For each red arrow shown inside the picture (for the steps 1 through 7) label what type of chemistry it is: nucleophilic attack, base catalysis, acid catalysis, enzyme substrate complex, redox reaction etc.





