Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. 2. 3. 4. 5. At critical condition of temperature and pressure for a real gas, what would be the value of 16 Z?
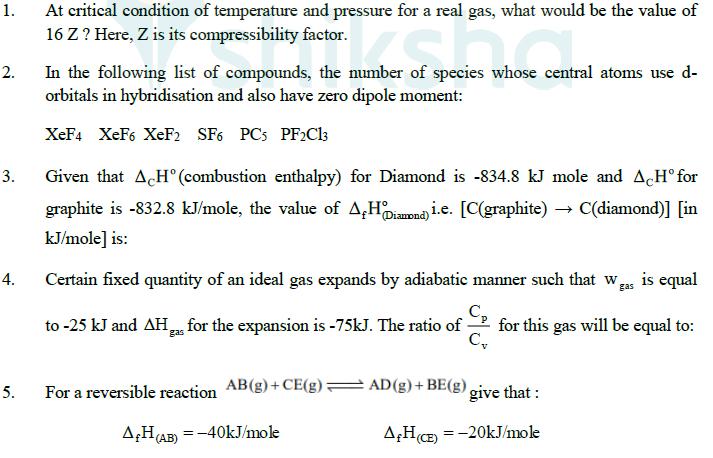

1. 2. 3. 4. 5. At critical condition of temperature and pressure for a real gas, what would be the value of 16 Z? Here, Z is its compressibility factor. of species whose In the following list of compounds, the number of species whose central atoms use d- orbitals in hybridisation and also have zero dipole moment: XeF4 XeF6 XeF2 SF6 PC5 PF2C13 Given that ACH(combustion enthalpy) for Diamond is -834.8 kJ mole and ACH for graphite is -832.8 kJ/mole, the value of A&H Diamond) i.e. [C(graphite) -> C(diamond)] [in kJ/mole] is: Certain fixed quantity of an ideal gas expands by adiabatic manner such that was is equal to -25 kJ and AH for the expansion is -75kJ. The ratio of gas C for this gas will be equal to: For a reversible reaction AB(g) +CE(g) AD(g) + BE(g) give that: AH (AB)=-40kJ/mole AH (CE) -20kJ/mole = AH (AD) =-30kJ/mole AHBE)=-10kJ/mole Entropy SAB = 5J/K_SCE =8J/K_SAD =15J/K SBE =18 J/K To equilibrium temperature for the reaction is 'T' K. What is the value of T 1000
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


