Answered step by step
Verified Expert Solution
Question
1 Approved Answer
16. Using the given data find value of resonance energy of benzene C6H6. AydgH of cyclohexene = -119 kJ/mole AhydgH of benzene = -206.5
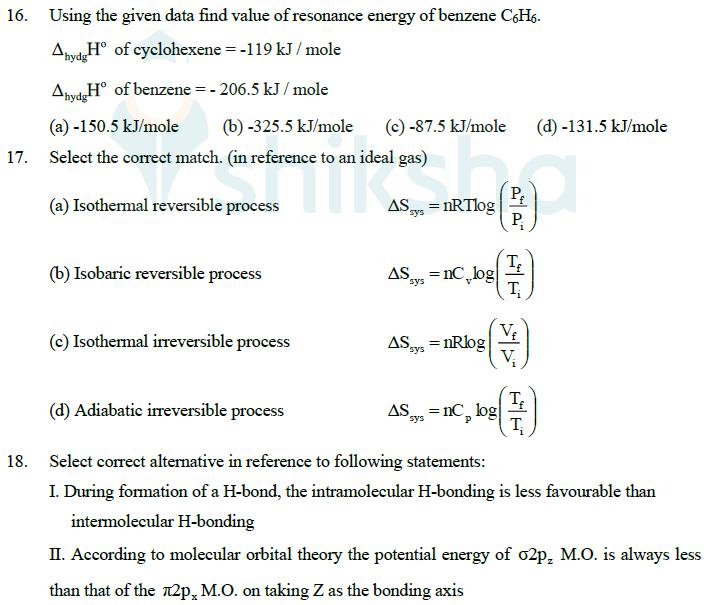
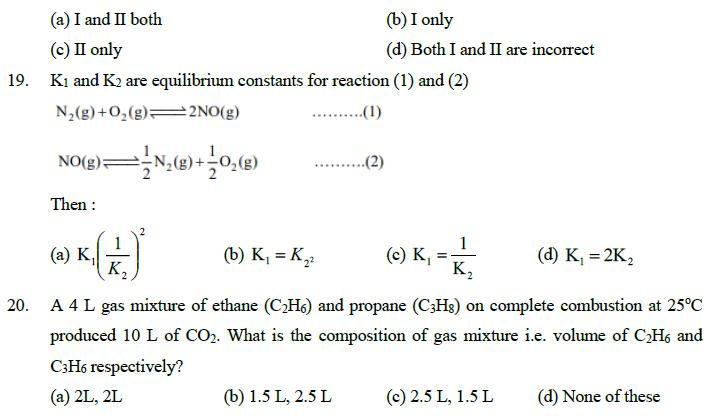
16. Using the given data find value of resonance energy of benzene C6H6. AydgH of cyclohexene = -119 kJ/mole AhydgH of benzene = -206.5 kJ/mole (a) -150.5 kJ/mole (b) -325.5 kJ/mole (c) -87.5 kJ/mole (d) -131.5 kJ/mole 17. Select the correct match. (in reference to an ideal gas) (a) Isothermal reversible process sys AS = nRTlog P (b) Isobaric reversible process AS = nC log sys T (c) Isothermal irreversible process AS = = nRlog sys (d) Adiabatic irreversible process sys AS=nc log T 18. Select correct alternative in reference to following statements: I. During formation of a H-bond, the intramolecular H-bonding is less favourable than intermolecular H-bonding II. According to molecular orbital theory the potential energy of 02p, M.O. is always less than that of the 2p, M.O. on taking Z as the bonding axis (a) I and II both (c) II only (b) I only (d) Both I and II are incorrect 19. Ki and K2 are equilibrium constants for reaction (1) and (2) N2(g) + O2(g) 2NO(g) ..........(1) NO(g) N2(g) + O2(g) ..... .(2) Then : (a) K, K (b) K = K22 (c) K = 1/4 (d) K = 2K2 K 20. A 4 L gas mixture of ethane (C2H6) and propane (C3H8) on complete combustion at 25C produced 10 L of CO2. What is the composition of gas mixture i.e. volume of CH6 and C3H6 respectively? (a) 2L, 2L (b) 1.5 L, 2.5 L (c) 2.5 L, 1.5 L (d) None of these
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


