Answered step by step
Verified Expert Solution
Question
1 Approved Answer
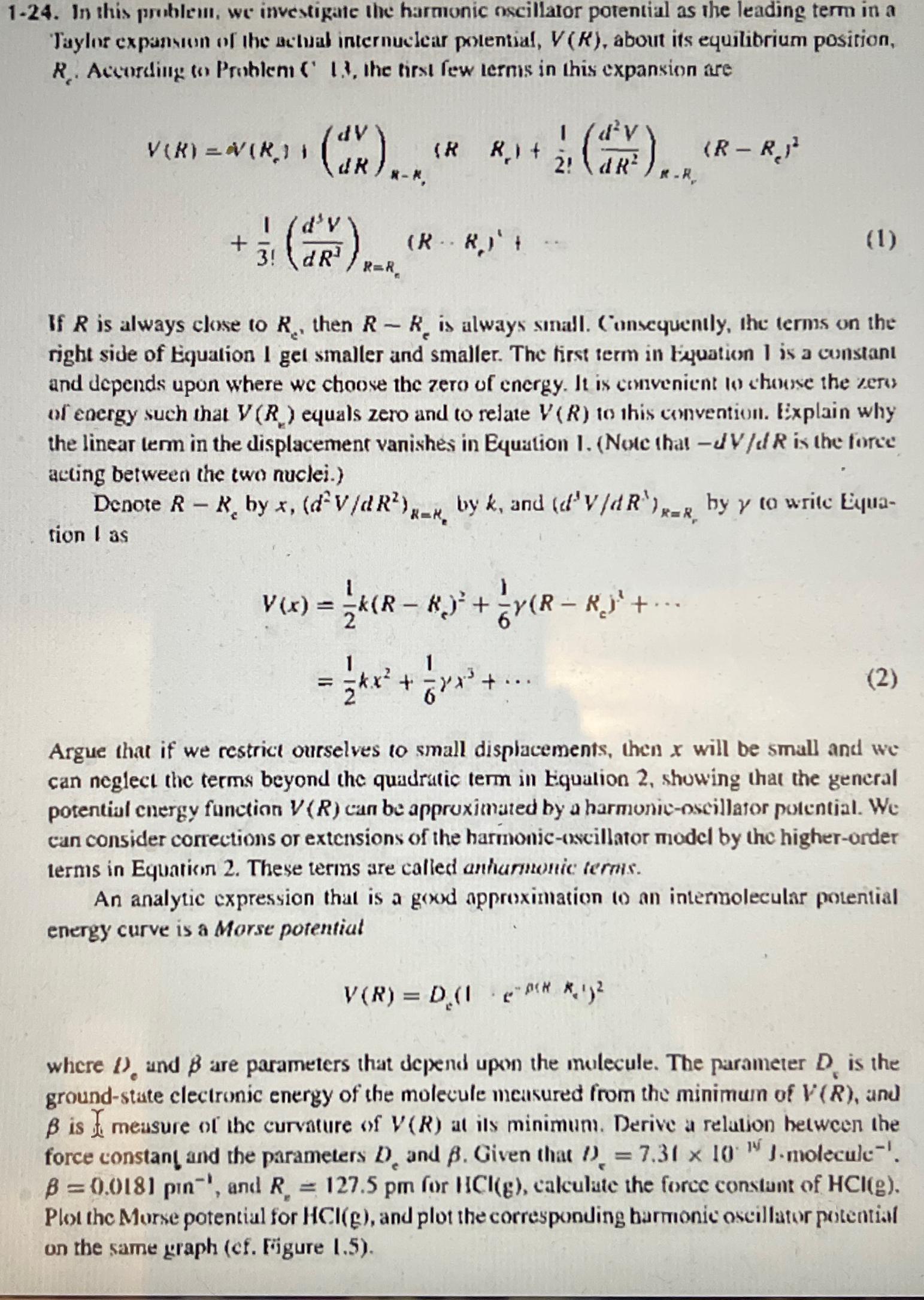
1 - 2 4 . In this problein, we investigate the harmonic nscillator potential as the leading term in a Taylor expansun of the actual
In this problein, we investigate the harmonic nscillator potential as the leading term in a Taylor expansun of the actual internuclear potential, about its equilibrium position, Accordilig Problent the tirst few terms in this expansion are
If is always close to then is always small. Consequently, the terms on the right side of Equation get smaller and smaller. The first term in Equation I is a cunstant and depends upon where we choose the zero of energy. It is comvenient to shouse the zers of energy such that equals zero and to relate to this convention. Explain why the linear term in the displacement vanishes in Equation I. Note that is the force acting between the two nuclei.
Denote by by and by to write Eyuation as
cdots
cdots
Argue that if we restrict ourselves to small displacements, then will be small and we can neglect the terms beyond the quadratic term in Equation showing that the general potential cnergy function can be approximated by a harmonicnscillator potential. We can consider corrections or extensions of the harmonicoscillator model by the higherorder terms in Equation These terms are called antiannonic terms.
An analytic expression that is a good approximation to an intermolecular potential energy curve is a Morse potentiat
where and are parameters that depend upon the mulecule. The parameter is the groundstate clectronic energy of the molecule measured from the minimum of and is hat meusure of the curvature of at is minimum. Derive a relation hetween the force constan! and the parameters and Given that and for calculate the force constant of Plot the Morse potential for and plot the corresponding harmonic oscillator potential on the same graph cf Frigure
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


