Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. 2. (c) Be(OH)2 (d) Sr(OH)2 Number of amphoteric compounds among the following is (a) BeO (B) Bao The stepwise formation of [Cu(NH)]* is
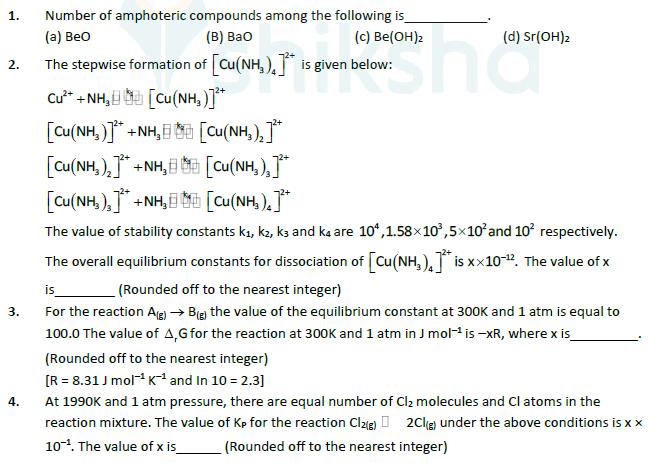
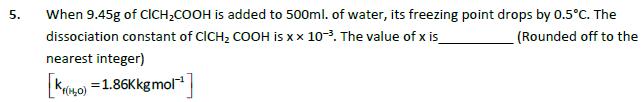
1. 2. (c) Be(OH)2 (d) Sr(OH)2 Number of amphoteric compounds among the following is (a) BeO (B) Bao The stepwise formation of [Cu(NH)]* is given below: C ANH, LE [Cu(NH, ) [Cu(NH)]+NH, [Cu(NH3)2]+NH, [Cu(NH3),]+NH, [Cu(NH3)2]* [Cu(NH3)3]* 3. 4. [Cu(NH).]* The value of stability constants k, kz, k3 and ka are 104,1.58103,5102 and 10 respectively. The overall equilibrium constants for dissociation of [Cu(NH3)4]** is xx10-12. The value of x is (Rounded off to the nearest integer) For the reaction A (g) Big) the value of the equilibrium constant at 300K and 1 atm is equal to 100.0 The value of A,G for the reaction at 300K and 1 atm in J mol- is -xR, where x is (Rounded off to the nearest integer) [R = 8.31 J mol K and In 10 = 2.3] At 1990K and 1 atm pressure, there are equal number of Cl molecules and Cl atoms in the reaction mixture. The value of Kp for the reaction Cl2(g) 2Cl(g) under the above conditions is x x 10. The value of x is (Rounded off to the nearest integer) 5. When 9.45g of CICH2COOH is added to 500ml. of water, its freezing point drops by 0.5C. The dissociation constant of CICH COOH is xx 10-. The value of x is (Rounded off to the nearest integer) K(0)=1.86kkgmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


