Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 2 The reform reaction between steam and gaseous methane (CH) produces synthesis gas, a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas
1 
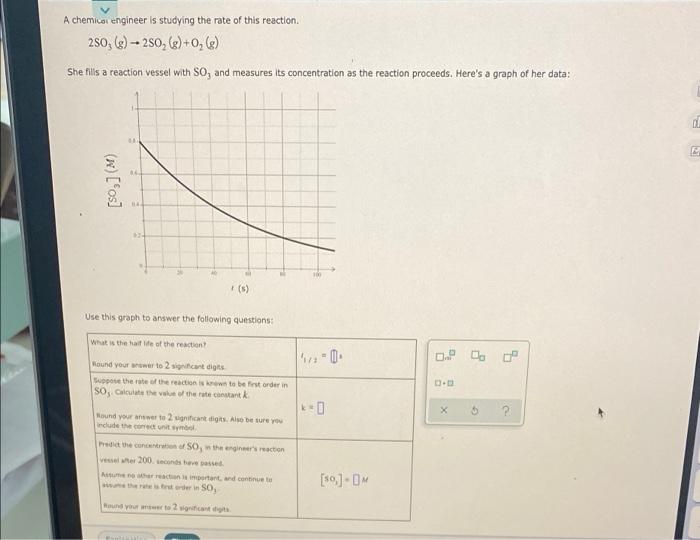
The reform reaction between steam and gaseous methane (CH) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. Suppose a chemical engineer studying a new catalyst for the reform reaction finds that 572. liters per second of methane are consumed when the reaction is run at 228. C and the methane is supplied at 0.69 atm. Calculate the rate at which dihydrogen is being produced. Give your answer in kilograms per second. Be sure your answer has the correct number of significant digits. 09 X U A chemical engineer is studying the rate of this reaction. 250, (e) - 250, (e+0,(e) - She fills a reaction vessel with So, and measures its concentration as the reaction proceeds. Here's a graph of her data: 0 (w) [fos] (6) Use this graph to answer the following questions What is the ide of the reaction Round your rower to 2 significant digits Support the rate of the reactions to be first order in SO, Calculate the value of the rate constant 0 X 5 2 Hound your answer to grant digits. Also be sure you dude the county Predict the concert SO, the engineer's action ver 200.csbeveel Manis intent and continue w the rest order in SO, und you to 2 canta [59]-OM 
2

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


