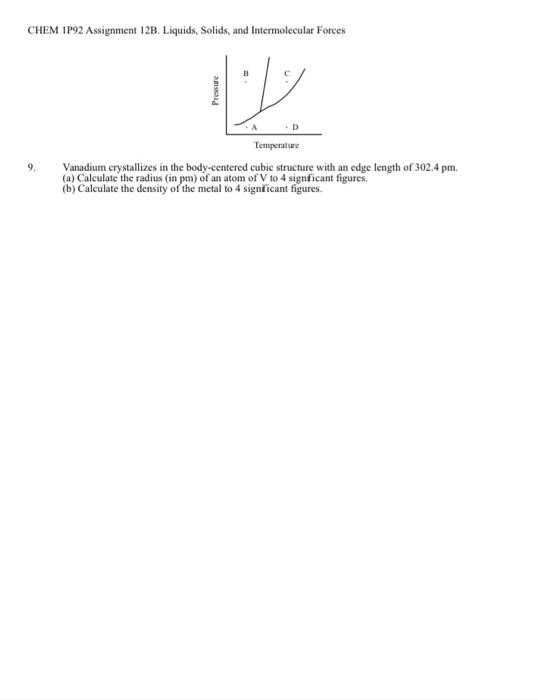
1. 20.00 1882 3. CHEM 1P92 Assignment 12A (Solutions). Version 8 179 Fill in the blanks in the table for aqueous solutions of the compounds shown. The density of the solution is in g/ml.. Abbreviations mis molality, Mass % is the mass percentage of the solute, Mole Frac. is the mole fraction of the solute, and M is molarity of the solute, Compound f.w. d. soln Mass% Mole Frac M , 17.03 0.9228 Na Cr:0 261.97 1.1260 0.7271 NH CON H. 60,06 1.0276 CHEM 1P92 Assignment 12B (Liquids, Solids, and Intermolecular Forces) Version # 179 Draw Lewis structures for the following compounds. Remember to enclose ions in square brackets. On your answer sheet, state the number of valence electrons in each compound. (a) NaNO(b)KO (C) CH,SH 2 List the most im portant (strongest) intermolecular force(s) that must be overcome to (a) melt solid Caso (b) vaporize liquid CHCI (c) remove water of hydration from Na CO - 10H0 Consider what happens when liquid CH OH dissolves in liquid CCI. a) What type of attractive forces must be overcome in the liquid CCI.? c) What type of attractive forces are important when CH OH dissolves in liquid CCI.? (a) Which of the ions K Rb Be will have the largest heat of hydration? Which the smallest? (b) of the two compounds Mg(NO) and Sr(NO), which has the stronger intermolecular forces? (c) of the two compounds cdi ,and CH OH, which has the stronger intermolecular forces! 5. Calculate the heat (in kJ) required to transform 62.50 g of carbon tetrachloride from a solid at a temperature of -23 C to a gas at 92 C. Report your answer to one decimal place. Data Molar mass of carbon tetrachloride, CCI.- 153.822 g/mol Melting point --23 C Boiling point = 76C Enthalpy of fusion - 2.56 kJ/mol Enthalpy of vaporization - 29.82 kJ/mol. Molar heat capacity of the liquid phase = 131.3 J/mol.K Molar heat capacity of the gas phase= 83.3 J/mol K. Make a plot of the heat transfer and temperature change for the system described in Question 5. Plot the temperature on the y axis and the heat added on the x-axis. Label the parts of the graph (solid melts, liquid is heated, etc.) Your graph will resemble Figure 11.39 in Tro, Fridgen and Shaw 7. A metal crystallizes in the face-centered cubic crystal structure with a unit cell edge of 3.89 x 10 cm. The density of the metal is 12.0 gcc. (a) What is the mass, in grams, of a single atom of this element? (b) What is the atomic weight of the element (g/mol). (c) What is the radius, in cm, of an atom of the element? As a substance is subjected to pressure and/or temperature changes, it may undergo condensation, deposition, freezing, melting, sublimation, or vaporization (a) Consider the phase diagram shown below and state the changes that take place upon going from points A to B to C to D. (b) is the transition from C to D exothermic or endothermic? 4. 6. 8 CHEM IP92 Assignment 123. Liquids, Solids, and Intermolecular Forces Press Q 9 Temperature Vanadium crystallizes in the body-centered cubic structure with an edge length of 302.4 pm. (a) Calculate the radius (in pm) of an atom of V to 4 significant figures. (b) Calculate the density of the metal to 4 significant figures. 1. 20.00 1882 3. CHEM 1P92 Assignment 12A (Solutions). Version 8 179 Fill in the blanks in the table for aqueous solutions of the compounds shown. The density of the solution is in g/ml.. Abbreviations mis molality, Mass % is the mass percentage of the solute, Mole Frac. is the mole fraction of the solute, and M is molarity of the solute, Compound f.w. d. soln Mass% Mole Frac M , 17.03 0.9228 Na Cr:0 261.97 1.1260 0.7271 NH CON H. 60,06 1.0276 CHEM 1P92 Assignment 12B (Liquids, Solids, and Intermolecular Forces) Version # 179 Draw Lewis structures for the following compounds. Remember to enclose ions in square brackets. On your answer sheet, state the number of valence electrons in each compound. (a) NaNO(b)KO (C) CH,SH 2 List the most im portant (strongest) intermolecular force(s) that must be overcome to (a) melt solid Caso (b) vaporize liquid CHCI (c) remove water of hydration from Na CO - 10H0 Consider what happens when liquid CH OH dissolves in liquid CCI. a) What type of attractive forces must be overcome in the liquid CCI.? c) What type of attractive forces are important when CH OH dissolves in liquid CCI.? (a) Which of the ions K Rb Be will have the largest heat of hydration? Which the smallest? (b) of the two compounds Mg(NO) and Sr(NO), which has the stronger intermolecular forces? (c) of the two compounds cdi ,and CH OH, which has the stronger intermolecular forces! 5. Calculate the heat (in kJ) required to transform 62.50 g of carbon tetrachloride from a solid at a temperature of -23 C to a gas at 92 C. Report your answer to one decimal place. Data Molar mass of carbon tetrachloride, CCI.- 153.822 g/mol Melting point --23 C Boiling point = 76C Enthalpy of fusion - 2.56 kJ/mol Enthalpy of vaporization - 29.82 kJ/mol. Molar heat capacity of the liquid phase = 131.3 J/mol.K Molar heat capacity of the gas phase= 83.3 J/mol K. Make a plot of the heat transfer and temperature change for the system described in Question 5. Plot the temperature on the y axis and the heat added on the x-axis. Label the parts of the graph (solid melts, liquid is heated, etc.) Your graph will resemble Figure 11.39 in Tro, Fridgen and Shaw 7. A metal crystallizes in the face-centered cubic crystal structure with a unit cell edge of 3.89 x 10 cm. The density of the metal is 12.0 gcc. (a) What is the mass, in grams, of a single atom of this element? (b) What is the atomic weight of the element (g/mol). (c) What is the radius, in cm, of an atom of the element? As a substance is subjected to pressure and/or temperature changes, it may undergo condensation, deposition, freezing, melting, sublimation, or vaporization (a) Consider the phase diagram shown below and state the changes that take place upon going from points A to B to C to D. (b) is the transition from C to D exothermic or endothermic? 4. 6. 8 CHEM IP92 Assignment 123. Liquids, Solids, and Intermolecular Forces Press Q 9 Temperature Vanadium crystallizes in the body-centered cubic structure with an edge length of 302.4 pm. (a) Calculate the radius (in pm) of an atom of V to 4 significant figures. (b) Calculate the density of the metal to 4 significant figures