Answered step by step
Verified Expert Solution
Question
1 Approved Answer
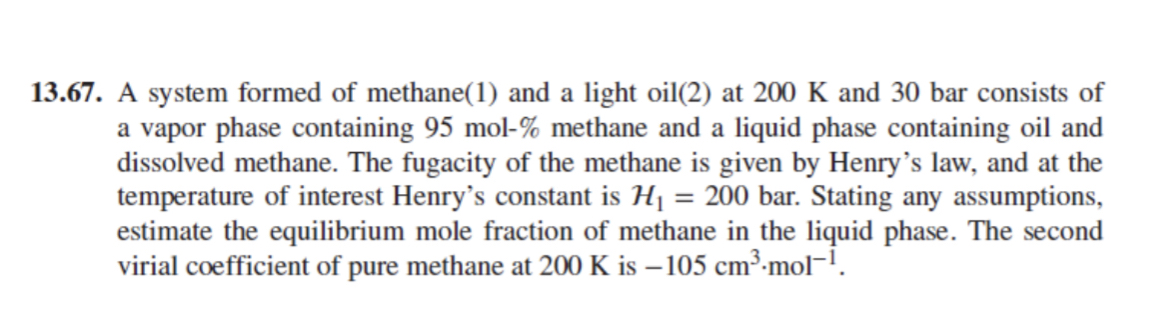
1 3 . 6 7 . A system formed of methane ( 1 ) and a light oil ( 2 ) at 2 0 0
A system formed of methane and a light oil at and bar consists of a vapor phase containing mol methane and a liquid phase containing oil and dissolved methane. The fugacity of the methane is given by Henry's law, and at the temperature of interest Henry's constant is bar. Stating any assumptions, estimate the equilibrium mole fraction of methane in the liquid phase. The second virial coefficient of pure methane at is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


