Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (30 points) Specific enthalpies and densities of argon at atmospheric pressure are given below: a. Calculate the specific enthalpy of argon at 240K and
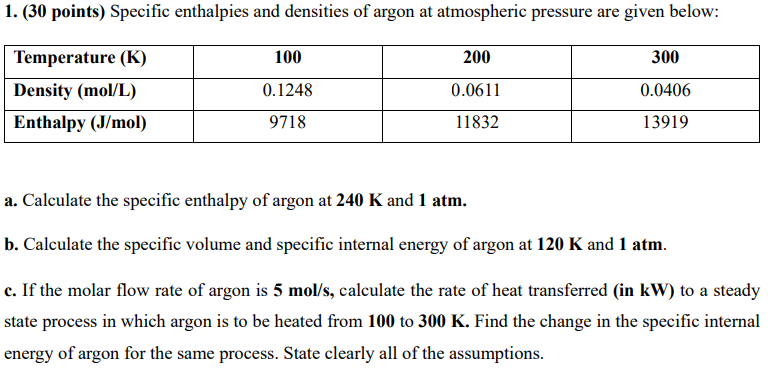 1. (30 points) Specific enthalpies and densities of argon at atmospheric pressure are given below: a. Calculate the specific enthalpy of argon at 240K and 1 atm. b. Calculate the specific volume and specific internal energy of argon at 120K and 1atm. c. If the molar flow rate of argon is 5mol/s, calculate the rate of heat transferred (in kW ) to a steady state process in which argon is to be heated from 100 to 300K. Find the change in the specific internal energy of argon for the same process. State clearly all of the assumptions
1. (30 points) Specific enthalpies and densities of argon at atmospheric pressure are given below: a. Calculate the specific enthalpy of argon at 240K and 1 atm. b. Calculate the specific volume and specific internal energy of argon at 120K and 1atm. c. If the molar flow rate of argon is 5mol/s, calculate the rate of heat transferred (in kW ) to a steady state process in which argon is to be heated from 100 to 300K. Find the change in the specific internal energy of argon for the same process. State clearly all of the assumptions Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


