Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (30 pts) To use in production of a fertilizer, it is desirable to obtain KNO3 with 99.5% purity from 1000kg/h of an aqueous solution
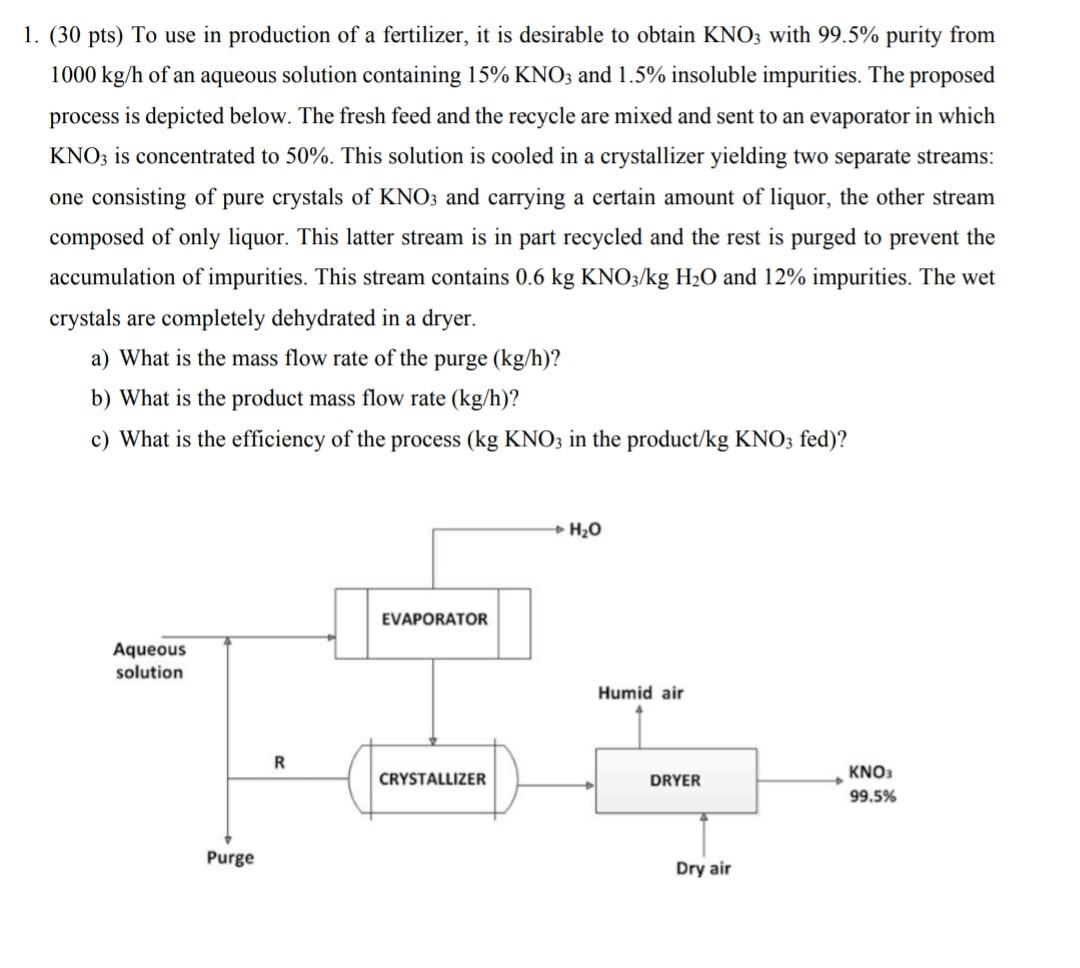
1. (30 pts) To use in production of a fertilizer, it is desirable to obtain KNO3 with 99.5% purity from 1000kg/h of an aqueous solution containing 15%KNO3 and 1.5% insoluble impurities. The proposed process is depicted below. The fresh feed and the recycle are mixed and sent to an evaporator in which KNO3 is concentrated to 50%. This solution is cooled in a crystallizer yielding two separate streams: one consisting of pure crystals of KNO3 and carrying a certain amount of liquor, the other stream composed of only liquor. This latter stream is in part recycled and the rest is purged to prevent the accumulation of impurities. This stream contains 0.6kgKNO/KgH2O and 12% impurities. The wet crystals are completely dehydrated in a dryer. a) What is the mass flow rate of the purge (kg/h) ? b) What is the product mass flow rate (kg/h) ? c) What is the efficiency of the process (kgKNO3 in the product/ kgKNO3 fed)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


