Answered step by step
Verified Expert Solution
Question
1 Approved Answer
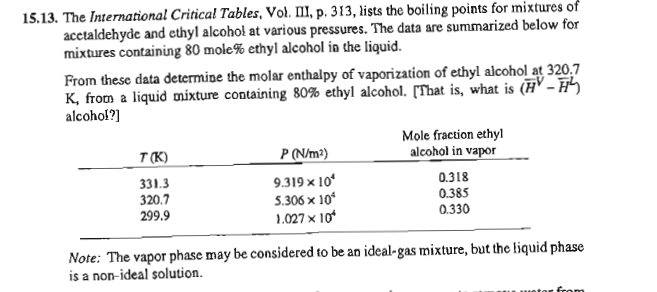
1 5 . 1 3 . The International Critical Tables, Vol. III, p . 3 1 3 , lists the boiling points for mixtures of
The International Critical Tables, Vol. III, p lists the boiling points for mixtures of
acetaldehyde and ethyl alcohol at various pressures. The data are summarized below for
mixtures containing mole ethyl alcohol in the liquid.
From these data determine the molar enthalpy of vaporization of ethyl alcohol at
from a liquid mixture containing ethyl alcohol. That is what is
alcohol?
Note: The vapor phase may be considered to be an idealgas mixture, but the liquid phase
is a nonideal solution. The correct answer is approximately roughly Jmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


